Related Research Articles

Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name biotin, borrowed from the German Biotin, derives from the Ancient Greek word βίοτος (bíotos; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white, needle-like crystalline solid.

The New York–Penn League (NYPL) was a Minor League Baseball league that operated in the northeastern United States from 1939 to 2020. Classified as a Class A Short Season league, its season started in June, after major-league teams signed their amateur draft picks to professional contracts, and ended in early September.

Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is used intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken orally as a treatment for severe Clostridium difficile colitis. When taken orally it is poorly absorbed.

Teichoic acids are bacterial copolymers of glycerol phosphate or ribitol phosphate and carbohydrates linked via phosphodiester bonds.

β-Alanine is a naturally occurring beta amino acid, which is an amino acid in which the amino group is attached to the β-carbon instead of the more usual α-carbon for alanine (α-alanine). The IUPAC name for β-alanine is 3-aminopropanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-αα-D-alanyl moiety of R-L-αα-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.

Interstate 88 (I-88) is an Interstate Highway located entirely within the US state of New York. Nominally signed as an east–west road as it has an even number, it extends for 117.75 miles (189.50 km) in a northeast–southwest direction from an interchange with I-81 north of the city of Binghamton to an interchange with the New York State Thruway (I-90) west of Schenectady. The freeway serves as an important connector route from the Capital District to Binghamton, Elmira, and Scranton, Pennsylvania. I-88 closely parallels NY 7, which was once the main route through the area.

Sir Gregory Paul Winter is a Nobel Prize-winning English molecular biologist best known for his work on the therapeutic use of monoclonal antibodies. His research career has been based almost entirely at the MRC Laboratory of Molecular Biology and the MRC Centre for Protein Engineering, in Cambridge, England.

Pseudopeptidoglycan is a major cell wall component of some Archaea that differs from bacterial peptidoglycan in chemical structure, but resembles bacterial peptidoglycan in function and physical structure. Pseudopeptidoglycan, in general, is only present in a few methanogenic archaea. The basic components are N-acetylglucosamine and N-acetyltalosaminuronic acid, which are linked by β-1,3-glycosidic bonds.

Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.

A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed.

Geobacillus stearothermophilus is a rod-shaped, Gram-positive bacterium and a member of the phylum Bacillota. The bacterium is a thermophile and is widely distributed in soil, hot springs, ocean sediment, and is a cause of spoilage in food products. It will grow within a temperature range of 30–75 °C. Some strains are capable of oxidizing carbon monoxide aerobically. It is commonly used as a challenge organism for sterilization validation studies and periodic check of sterilization cycles. The biological indicator contains spores of the organism on filter paper inside a vial. After sterilizing, the cap is closed, an ampule of growth medium inside of the vial is crushed and the whole vial is incubated. A color and/or turbidity change indicates the results of the sterilization process; no change indicates that the sterilization conditions were achieved, otherwise the growth of the spores indicates that the sterilization process has not been met. Fluorescent-tagged strains, known as rapid-read BIs, are becoming more common to verify sterilization, since the visible fluorescence appears in about one-tenth the time needed for pH-indicator color change and an inexpensive light sensor can detect the growing colonies.

The transsulfuration pathway is a metabolic pathway involving the interconversion of cysteine and homocysteine through the intermediate cystathionine. Two transsulfurylation pathways are known: the forward and the reverse.
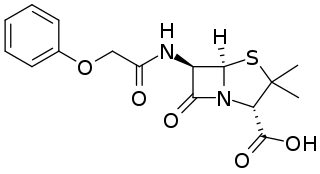
Phenoxymethylpenicillin, also known as penicillin V (PcV) and penicillin VK, is an antibiotic useful for the treatment of a number of bacterial infections. Specifically it is used for the treatment of strep throat, otitis media, and cellulitis. It is also used to prevent rheumatic fever and to prevent infections following removal of the spleen. It is given by mouth.

In enzymology, an alanine racemase is an enzyme that catalyzes the chemical reaction
Radical SAM enzymes belong to a superfamily of enzymes that use an iron-sulfur cluster (4Fe-4S) to reductively cleave S-adenosyl-L-methionine (SAM) to generate a radical, usually a 5′-deoxyadenosyl radical (5'-dAdo), as a critical intermediate. These enzymes utilize this radical intermediate to perform diverse transformations, often to functionalize unactivated C-H bonds. Radical SAM enzymes are involved in cofactor biosynthesis, enzyme activation, peptide modification, post-transcriptional and post-translational modifications, metalloprotein cluster formation, tRNA modification, lipid metabolism, biosynthesis of antibiotics and natural products etc. The vast majority of known radical SAM enzymes belong to the radical SAM superfamily, and have a cysteine-rich motif that matches or resembles CxxxCxxC. Radical SAM enzymes comprise the largest superfamily of metal-containing enzymes.
Muramoylpentapeptide carboxypeptidase is an enzyme. This enzyme catalyses the following chemical reaction.
Caldibacillus is a facultative anaerobe genus of bacteria that stains Gram-positive from the family of Bacillaceae. The type species of this genus is Caldibacillus debilis.

Elsie Mae Frost was an American educator. She earned her B.S. and M.A. from Teachers College, Columbia University. Her legacy is detailed in Life with Elsie by James A. Frost. Additionally, the College at Oneonta established a scholarship in her honor.
References
- 1 2 Frost 2006, pp. 162.
- ↑ "NEASC 2016 Self-Study Report" (PDF). capitalcc.edu. Retrieved 2024-02-15.
- ↑ "Frost Given State Post" . The Daily Star (Oneonta) . 12 June 1964. p. 5. Archived from the original on 15 June 2024. Retrieved 15 June 2024– via Newspapers.com.
- 1 2 "Structural studies of the enzyme D-alanyl-D-alanine carboxypeptidase from Bacillus stearothermophilus". 1994. Archived from the original on 15 June 2024. Retrieved 15 June 2024– via storrs.primo.exlibrisgroup.com.
- ↑ "The biosynthesis of a biotin compound containing ⁷⁵Se". 1977. Archived from the original on 15 June 2024. Retrieved 14 June 2024– via suny-bin.primo.exlibrisgroup.com.
- ↑ "The ORB: Open Repository at Binghamton, Masters Thesis". 1977. Archived from the original on 3 July 2024. Retrieved 3 July 2024– via orb.binghamton.edu.
- ↑ "Frost-Naleski Marriage Announcement" . Hartford Courant . 13 June 1982. p. 122. Archived from the original on 15 June 2024. Retrieved 15 June 2024– via Newspapers.com.
Works Cited
Books
- Frost, James A. (2006). Life with Elsie. Plainville, Connecticut: The Briarwood Printing Co.