
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide, cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
In chemistry, homogeneous catalysis is catalysis where the catalyst is in same phase as reactants, principally by a soluble catalyst a in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts.
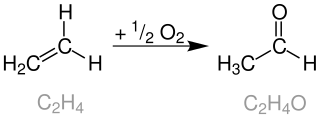
The Wacker process or the Hoechst-Wacker process refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride and copper(II) chloride as the catalyst. This chemical reaction was one of the first homogeneous catalysis with organopalladium chemistry applied on an industrial scale.

In organic chemistry, olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.

Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes. The reaction is related to olefin metathesis.
Alkane metathesis is a class of chemical reaction in which an alkane is rearranged to give a longer or shorter alkane product. It is similar to olefin metathesis, except that olefin metathesis cleaves and recreates a carbon-carbon double bond, but alkane metathesis operates on a carbon-carbon single bond.

Yves Chauvin was a French chemist and Nobel Prize laureate. He was honorary research director at the Institut français du pétrole and a member of the French Academy of Science. He was known for his work for deciphering the process of olefin metathesis for which he was awarded the 2005 Nobel Prize in Chemistry along with Robert H. Grubbs and Richard R. Schrock.
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.

Crabtree's catalyst is an organoiridium compound with the formula [C8H12IrP(C6H11)3C5H5N]PF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert H. Crabtree. This air stable orange solid is commercially available and known for its directed hydrogenation to give trans stereoselectivity with respective of directing group.
In organic chemistry and organometallic chemistry, carbon–hydrogen bond activation is a type of organic reaction in which a carbon–hydrogen bond is cleaved and replaced with a C−X bond. Some authors further restrict the term C–H activation to reactions in which a C–H bond, one that is typically considered to be "unreactive", interacts with a transition metal center M, resulting in its cleavage and the generation of an organometallic species with an M–C bond. The intermediate of this step could then undergo subsequent reactions with other reagents, either in situ or in a separate step, to produce the functionalized product.

Amir H. Hoveyda is an American organic chemist and professor of chemistry at Boston College, and held the position of department chair until 2018. In 2019, he embarked as researcher at the Institute of Science and Supramolecular Engineering at University of Strasbourg.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
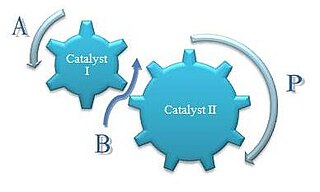
Concurrent tandem catalysis (CTC) is a technique in chemistry where multiple catalysts produce a product otherwise not accessible by a single catalyst. It is usually practiced as homogeneous catalysis. Scheme 1 illustrates this process. Molecule A enters this catalytic system to produce the comonomer, B, which along with A enters the next catalytic process to produce the final product, P. This one-pot approach can decrease product loss from isolation or purification of intermediates. Reactions with relatively unstable products can be generated as intermediates because they are only transient species and are immediately used in a consecutive reaction.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.
Methane functionalization is the process of converting methane in its gaseous state to another molecule with a functional group, typically methanol or acetic acid, through the use of transition metal catalysts.
Karen Ila Goldberg is an American chemist, currently the Vagelos Professor of Energy Research at University of Pennsylvania. Goldberg is most known for her work in inorganic and organometallic chemistry. Her most recent research focuses on catalysis, particularly on developing catalysts for oxidation, as well as the synthesis and activation of molecular oxygen. In 2018, Goldberg was elected to the National Academy of Sciences.
In organic chemistry, hydrovinylation is the formal insertion of an alkene into the C-H bond of ethylene. The more general reaction, hydroalkenylation, is the formal insertion of an alkene into the C-H bond of any terminal alkene. The reaction is catalyzed by metal complexes. A representative reaction is the conversion of styrene and ethylene to 3-phenybutene:
Olefin Conversion Technology, also called the Phillips Triolefin Process, is the industrial process that interconverts propylene with ethylene and 2-butenes. The process is also called the ethylene to propylene (ETP) process. In ETP, ethylene is dimerized to 1-butene, which is isomerized to 2-butenes. The 2-butenes are then subjected to metathesis with ethylene.










