
A scar is an area of fibrous tissue that replaces normal skin after an injury. Scars result from the biological process of wound repair in the skin, as well as in other organs, and tissues of the body. Thus, scarring is a natural part of the healing process. With the exception of very minor lesions, every wound results in some degree of scarring. An exception to this are animals with complete regeneration, which regrow tissue without scar formation.

In biology, the extracellular matrix (ECM), also called intercellular matrix, is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.

With physical trauma or disease suffered by an organism, healing involves the repairing of damaged tissue(s), organs and the biological system as a whole and resumption of (normal) functioning. Medicine includes the process by which the cells in the body regenerate and repair to reduce the size of a damaged or necrotic area and replace it with new living tissue. The replacement can happen in two ways: by regeneration in which the necrotic cells are replaced by new cells that form "like" tissue as was originally there; or by repair in which injured tissue is replaced with scar tissue. Most organs will heal using a mixture of both mechanisms.
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily.
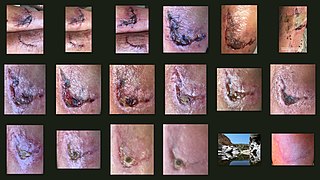
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.
Lawrence S.B. Goldstein is a professor of cellular and molecular medicine at University of California, San Diego and investigator with the Howard Hughes Medical Institute. He receives grant funding from the NIH, the Johns Hopkins ALS Center, the HighQ Foundation, and the California Institute for Regenerative Medicine. In 2020 he was elected to the National Academy of Sciences.
Collagenases are enzymes that break the peptide bonds in collagen. They assist in destroying extracellular structures in the pathogenesis of bacteria such as Clostridium. They are considered a virulence factor, facilitating the spread of gas gangrene. They normally target the connective tissue in muscle cells and other body organs.
Microbial collagenase is an enzyme. This enzyme catalyses the following chemical reaction

Keith Roberts Porter was a Canadian-American cell biologist. He created pioneering biology techniques and research using electron microscopy of cells. Porter also contributed to the development of other experimental methods for cell culture and nuclear transplantation. He was also responsible for naming the endoplasmic reticulum, conducting work on the 9 + 2 microtubule structure in the axoneme of cilia, and coining the term "microtrabecular lattice." In collaborations with other scientists, he contributed to the understanding of cellular structures and concepts such as compartmentalization, flagella, centrioles, fibrin, collagen, T-tubules and sarcoplasmic reticulum. He also introduced microtome cutting.
Alexander Rich was an American biologist and biophysicist. He was the William Thompson Sedgwick Professor of Biophysics at MIT and Harvard Medical School. Rich earned an A.B. and an M.D. from Harvard University. He was a post-doc of Linus Pauling. During this time he was a member of the RNA Tie Club, a social and discussion group which attacked the question of how DNA encodes proteins. He had over 600 publications to his name.
Interstitial collagenase, also known as fibroblast collagenase, and matrix metalloproteinase-1(MMP-1) is an enzyme that in humans is encoded by the MMP1 gene. The gene is part of a cluster of MMP genes which localize to chromosome 11q22.3. MMP-1 was the first vertebrate collagenase both purified to homogeneity as a protein, and cloned as a cDNA. MMP-1 has an estimated molecular mass of 54 kDa.

Collagenase 3 is an enzyme that in humans is encoded by the MMP13 gene. It is a member of the matrix metalloproteinase (MMP) family. Like most MMPs, it is secreted as an inactive pro-form. MMP-13 has an predicted molecular weight around 54 kDa. It is activated once the pro-domain is cleaved, leaving an active enzyme composed of the catalytic domain and the hemopexin-like domain PDB: 1PEX. Although the actual mechanism has not been described, the hemopexin domain participates in collagen degradation, the catalytic domain alone being particularly inefficient in collagen degradation. During embryonic development, MMP-13 is expressed in the skeleton as required for restructuring the collagen matrix for bone mineralization. In pathological situations it is highly overexpressed; this occurs in human carcinomas, rheumatoid arthritis and osteoarthritis.

Victor R. Ambros is an American developmental biologist who discovered the first known microRNA (miRNA). He is a professor at the University of Massachusetts Medical School in Worcester, Massachusetts.
Thomas Dean Pollard is a prominent educator, cell biologist and biophysicist whose research focuses on understanding cell motility through the study of actin filaments and myosin motors. He is Sterling Professor of Molecular, Cellular & Developmental Biology and a professor of cell biology and molecular biophysics & biochemistry at Yale University. He was dean of Yale's Graduate School of Arts and Sciences from 2010 to 2014, and president of the Salk Institute for Biological Studies from 1996 to 2001.
Brian Keith Hall is the George S. Campbell Professor of Biology and University Research Professor Emeritus at Dalhousie University in Halifax, Nova Scotia. Hall has researched and extensively written on bone and cartilage formation in developing vertebrate embryos. He is an active participant in the evolutionary developmental biology (EVO-DEVO) debate on the nature and mechanisms of animal body plan formation. Hall has proposed that the neural crest tissue of vertebrates may be viewed as a fourth embryonic germ layer. As such, the neural crest - in Hall's view - plays a role equivalent to that of the endoderm, mesoderm, and ectoderm of bilaterian development and is a definitive feature of vertebrates. As such, vertebrates are the only quadroblastic, rather than triploblastic bilaterian animals. In vertebrates the neural crest serves to integrate the somatic division and visceral division together via a wide range novel vertebrate tissues.

Donald E. Ingber is an American cell biologist and bioengineer. He is the founding director of the Wyss Institute for Biologically Inspired Engineering at Harvard University, the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children's Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences. He is also a member of the American Institute for Medical and Biological Engineering, the National Academy of Engineering, the National Academy of Medicine, the National Academy of Inventors, and the American Academy of Arts and Sciences.

Elizabeth Dexter "Betty" Hay was an American cell and developmental biologist. She was best known for her research in limb regeneration, the role of the extracellular matrix (ECM) in cell differentiation, and epithelial-mesenchymal transitions (EMT). Hay led many research teams in discovering new findings in these related fields, which led her to obtain several high honors and awards for her work. Hay primarily worked with amphibians during her years of limb regeneration work and then moved onto avian epithelia for research on the ECM and EMT. Hay was thrilled by the introduction of transmission electron microscopy (TEM) during her lifetime, which aided her in many of her findings throughout her career. Moreover, Hay was a huge advocate of women in science during her lifetime.
Scar free healing is the process by which significant injuries can heal without permanent damage to the tissue the injury has affected. In most healing, scars form due to the fibrosis and wound contraction, however in scar free healing, tissue is completely regenerated. During the 1990s, published research on the subject increased; it is a relatively recent term in the literature. Scar free healing occurs in foetal life but the ability progressively diminishes into adulthood. In other animals such as amphibians, however, tissue regeneration occurs, for example as skin regeneration in the adult axolotl.
Patricia Kilroy Donahoe is an American pediatric surgeon and a leading researcher in the field of developmental biology and oncology. She was the president of the American Pediatric Surgical Association from 2006 to 2007. She currently serves as the director of pediatric surgical research laboratories and chief emerita of pediatric surgical services at Massachusetts General Hospital.
Lathyrism is a condition caused by eating certain legumes of the genus Lathyrus. There are three types of lathyrism: neurolathyrism, osteolathyrism, and angiolathyrism, all of which are incurable, differing in their symptoms and in the body tissues affected.








