Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are irreversible acetylcholinesterase inhibitors used as poison.
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules such as proteins and nucleic acids, which is overseen by the Worldwide Protein Data Bank (wwPDB). These structural data are obtained and deposited by biologists and biochemists worldwide through the use of experimental methodologies such as X-ray crystallography, NMR spectroscopy, and, increasingly, cryo-electron microscopy. All submitted data are reviewed by expert biocurators and, once approved, are made freely available on the Internet under the CC0 Public Domain Dedication. Global access to the data is provided by the websites of the wwPDB member organisations.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:

Pyridostigmine is a medication used to treat myasthenia gravis and underactive bladder. It is also used together with atropine to end the effects of neuromuscular blocking medication of the non-depolarizing type. It is also used off-label to treat some forms of Postural orthostatic tachycardia syndrome. It is typically given by mouth but can also be used by injection. The effects generally begin within 45 minutes and last up to 4 hours.
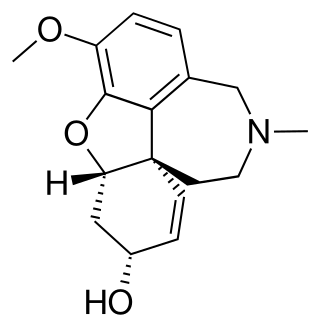
Galantamine is a type of acetylcholinesterase inhibitor. It is an alkaloid extracted from the bulbs and flowers of Galanthus nivalis, Galanthus caucasicus, Galanthus woronowii, and other members of the family Amaryllidaceae, such as Narcissus (daffodil), Leucojum aestivum (snowflake), and Lycoris including Lycoris radiata. It can also be produced synthetically.

Huperzine A is a naturally-occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a 2013 meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine (ACh) by the enzyme acetylcholinesterase. It is also an antagonist of the NMDA-receptor. It is commonly available over the counter as a nutritional supplement and marketed as a memory and concentration enhancer.
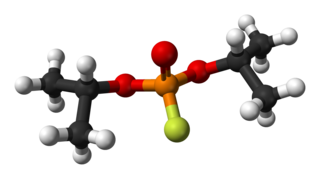
Diisopropyl fluorophosphate (DFP) or Isoflurophate is an oily, colorless liquid with the chemical formula C6H14FO3P. It is used in medicine and as an organophosphorus insecticide. It is stable, but undergoes hydrolysis when subjected to moisture.
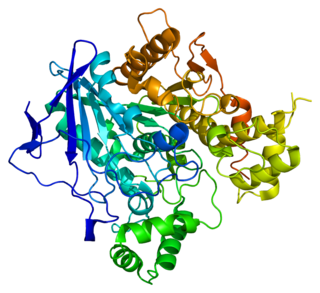
Butyrylcholinesterase, also known asBChE, BuChE, BuChase, pseudocholinesterase, or plasma (cholin)esterase, is a nonspecific cholinesterase enzyme that hydrolyses many different choline-based esters. In humans, it is made in the liver, found mainly in blood plasma, and encoded by the BCHE gene.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:

Carboxylesterase, type B is a family of evolutionarily related proteins that belongs to the superfamily of proteins with the Alpha/beta hydrolase fold.

The alpha/beta hydrolase superfamily is a superfamily of hydrolytic enzymes of widely differing phylogenetic origin and catalytic function that share a common fold. The core of each enzyme is an alpha/beta-sheet, containing 8 beta strands connected by 6 alpha helices. The enzymes are believed to have diverged from a common ancestor, retaining little obvious sequence similarity, but preserving the arrangement of the catalytic residues. All have a catalytic triad, the elements of which are borne on loops, which are the best-conserved structural features of the fold.

Paraoxonases are a family of mammalian enzymes with aryldialkylphosphatase activity. There are three paraoxonase isozymes, which were originally discovered for their involvement in the hydrolysis of organophosphates.

Proteopedia is a wiki, 3D encyclopedia of proteins and other molecules.

Cholinesterase inhibitors (ChEIs), also known as anti-cholinesterase, are chemicals that prevent the breakdown of the neurotransmitter acetylcholine or butyrylcholine by cholinesterase. This increases the amount of the acetylcholine or butyrylcholine in the synaptic cleft that can bind to muscarinic receptors, nicotinic receptors and others. This group of inhibitors is divided into two subgroups, acetylcholinesterase inhibitors (AChEIs) and butyrylcholinesterase inhibitors (BChEIs).

Rivastigmine, sold under the brand name Exelon among others, is an acetylcholinesterase inhibitor used for the treatment of dementia associated with Alzheimer's disease and with Parkinson's disease. Rivastigmine can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects, which typically include nausea and vomiting.

In molecular biology, protein fold classes are broad categories of protein tertiary structure topology. They describe groups of proteins that share similar amino acid and secondary structure proportions. Each class contains multiple, independent protein superfamilies.

Hermona Soreq is an Israeli professor of Molecular Neuroscience at The Hebrew University of Jerusalem. Best known for her work on the signaling of acetylcholine and its relevance in stress responses and neurodegenerative diseases such as Parkinson's and Alzheimer's.

Fasciculins are a class of toxic proteins found in certain snake venoms, notably some species of mamba. Investigations have revealed distinct forms in some green mamba venoms, in particular FAS1 and FAS2 Fasciculins are so called because they cause intense fasciculation in muscle fascicles of susceptible organisms, such as the preferred prey of the snakes. This effect helps to incapacitate the muscles, either killing the prey, or paralysing it so that the snake can swallow it.

TMTFA is an extremely potent acetylcholinesterase inhibitor. As a transition state analog of acetylcholinesterase, TMTFA is able to inhibit acetylcholinesterase at extremely low concentrations, making it one of the most potent acetylcholinesterase inhibitors known.
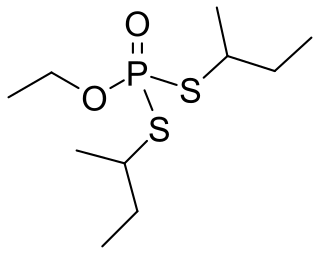
Cadusafos is a chemical insecticide and nematicide often used against parasitic nematode populations. The compound acts as a acetylcholinesterase inhibitor. It belongs the chemical class of synthetic organic thiophosphates and it is a volatile and persistent clear liquid. It is used on food crops such as tomatoes, bananas and chickpeas. It is currently not approved by the European Commission for use in the EU. Exposure can occur through inhalation, ingestion or contact with the skin. The compound is highly toxic to nematodes, earthworms and birds but poses no carcinogenic risk to humans.


















