Related Research Articles

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

A fuel cell is an electrochemical cell that converts the chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.
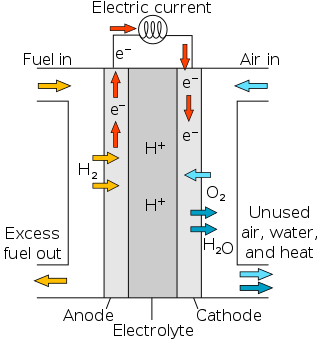
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.
A thermionic converter consists of a hot electrode which thermionically emits electrons over a potential energy barrier to a cooler electrode, producing a useful electric power output. Caesium vapor is used to optimize the electrode work functions and provide an ion supply to neutralize the electron space charge.
An atomic battery, nuclear battery, radioisotope battery or radioisotope generator is a device which uses energy from the decay of a radioactive isotope to generate electricity. Like nuclear reactors, they generate electricity from nuclear energy, but differ in that they do not use a chain reaction. Although commonly called batteries, they are technically not electrochemical and cannot be charged or recharged. They are very costly, but have an extremely long life and high energy density, and so they are typically used as power sources for equipment that must operate unattended for long periods of time, such as spacecraft, pacemakers, underwater systems and automated scientific stations in remote parts of the world.
Micro combined heat and power, micro-CHP, µCHP or mCHP is an extension of the idea of cogeneration to the single/multi family home or small office building in the range of up to 50 kW. Usual technologies for the production of heat and power in one common process are e.g. internal combustion engines, micro gas turbines, stirling engines or fuel cells.
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.

Électrolysis of
An alkali-metal thermal-to-electric converter is a thermally regenerative electrochemical device for the direct conversion of heat to electrical energy. It is characterized by high potential efficiencies and no moving parts except for the working fluid, which make it a candidate for space power applications.
A hydrogen compressor is a device that increases the pressure of hydrogen by reducing its volume resulting in compressed hydrogen or liquid hydrogen.

Reformed Methanol Fuel Cell (RMFC) or Indirect Methanol Fuel Cell (IMFC) systems are a subcategory of proton-exchange fuel cells where, the fuel, methanol (CH3OH), is reformed, before being fed into the fuel cell.
An automotive thermoelectric generator (ATEG) is a device that converts some of the waste heat of an internal combustion engine (IC) into electricity using the Seebeck Effect. A typical ATEG consists of four main elements: A hot-side heat exchanger, a cold-side heat exchanger, thermoelectric materials, and a compression assembly system. ATEGs can convert waste heat from an engine's coolant or exhaust into electricity. By reclaiming this otherwise lost energy, ATEGs decrease fuel consumed by the electric generator load on the engine. However, the cost of the unit and the extra fuel consumed due to its weight must be also considered.
Gas diffusion electrodes (GDE) are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase.

A membrane electrode assembly (MEA) is an assembled stack of proton-exchange membranes (PEM) or alkali anion exchange membrane (AAEM), catalyst and flat plate electrode used in fuel cells and electrolyzers.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
An electrochemical hydrogen compressor is a hydrogen compressor where hydrogen is supplied to the anode, and compressed hydrogen is collected at the cathode with an exergy efficiency up to and even beyond 80% for pressures up to 10,000 psi or 700 bars.
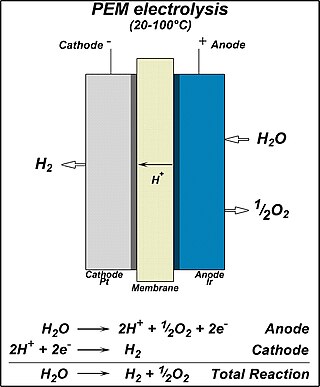
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.

An internal combustion engine is a heat engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons, turbine blades, a rotor, or a nozzle. This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to.

In electrochemistry, a thermogalvanic cell is a kind of galvanic cell in which heat is employed to provide electrical power directly. These cells are electrochemical cells in which the two electrodes are deliberately maintained at different temperatures. This temperature difference generates a potential difference between the electrodes. The electrodes can be of identical composition and the electrolyte solution homogeneous. This is usually the case in these cells. This is in contrast to galvanic cells in which electrodes and/or solutions of different composition provide the electromotive potential. As long as there is a difference in temperature between the electrodes a current will flow through the circuit. A thermogalvanic cell can be seen as analogous to a concentration cell but instead of running on differences in the concentration/pressure of the reactants they make use of differences in the "concentrations" of thermal energy. The principal application of thermogalvanic cells is the production of electricity from low-temperature heat sources. Their energetic efficiency is low, in the range of 0.1% to 1% for conversion of heat into electricity.
High Temperature Proton Exchange Membrane fuel cells (HT-PEMFC), also known as High Temperature Polymer Electrolyte Membrane fuel cells, are a type of PEM fuel cells which can be operated at temperatures between 120 and 200°C. HT-PEM fuel cells are used for both stationary and portable applications. The HT-PEM fuel cell is usually supplied with hydrogen-rich gas like reformate gas formed by reforming of methanol, ethanol, natural gas or LPG.
References
- ↑ "The Johnson Thermoelectric Energy Conversion System (JTEC)" (article). Bright Hub. 2010-09-26. Retrieved 2010-09-26.
- ↑ Johnson, Lonnie G. (25-28 February 2019). "Johnson Thermo-Electrochemical Converter (JTEC) as a Heat to Electric Generator for Nuclear Power Systems," Nuclear and Emerging Technologies for Space, American Nuclear Society Topical Meeting, Richland, WA. Retrieved 2021-10-22.
- ↑ Ward, Logan (1 January 2008). "Super Soaker Inventor Aims to Cut Solar Costs in Half" (article). Popular Mechanics . Retrieved 2010-09-18.
- ↑ Ward, Logan (1 October 2010). "Shooting for the Sun" (article). The Atlantic . Retrieved 1 October 2010.
- ↑ Johnson, Lonnie G. (29 January 2009). "High efficiency solid state engine". PARC. Retrieved 2010-09-18.
- ↑ "Johnson Thermo-Electrochemical Converter System" (Company Website). Johnson Electro-Mechanical Systems. Retrieved 2010-09-18.