
Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. In plants, terpenes and terpenoids are important mediators of ecological interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control.
Linoleic acid (LA) is an organic compound with the formula HOOC(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n−6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.
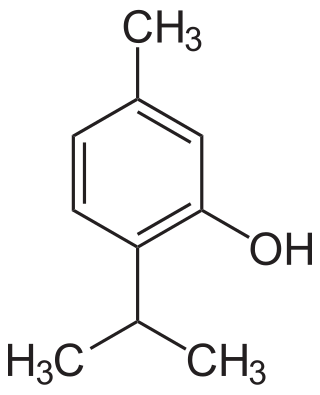
Thymol, C10H14O, is a natural monoterpenoid phenol derivative of p-Cymene, isomeric with carvacrol, found in oil of thyme, and extracted from Thymus vulgaris, ajwain, and various other plants as a white crystalline substance of a pleasant aromatic odor and strong antiseptic properties. Thymol also provides the distinctive, strong flavor of the culinary herb thyme, also produced from T. vulgaris. Thymol is only slightly soluble in water at neutral pH, but it is extremely soluble in alcohols and other organic solvents. It is also soluble in strongly alkaline aqueous solutions due to deprotonation of the phenol. Its dissociation constant (pKa) is 10.59±0.10. Thymol absorbs maximum UV radiation at 274 nm.

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.
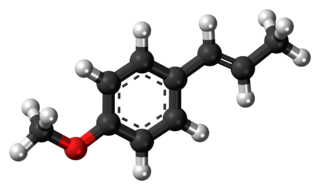
Anethole is an organic compound that is widely used as a flavoring substance. It is a derivative of the aromatic compound allylbenzene and occurs widely in the essential oils of plants. It is in the class of phenylpropanoid organic compounds. It contributes a large component of the odor and flavor of anise and fennel, anise myrtle (Myrtaceae), liquorice (Fabaceae), magnolia blossoms, and star anise (Schisandraceae). Closely related to anethole is its isomer estragole, which is abundant in tarragon (Asteraceae) and basil (Lamiaceae), and has a flavor reminiscent of anise. It is a colorless, fragrant, mildly volatile liquid. Anethole is only slightly soluble in water but exhibits high solubility in ethanol. This trait causes certain anise-flavored liqueurs to become opaque when diluted with water; this is called the ouzo effect.

Pulegone is a naturally occurring organic compound obtained from the essential oils of a variety of plants such as Nepeta cataria (catnip), Mentha piperita, and pennyroyal. It is classified as a monoterpene.

Caryophyllene, more formally (−)-β-caryophyllene (BCP), is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, copaiba, rosemary, and hops. It is usually found as a mixture with isocaryophyllene and α-humulene, a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, as well as a trans-double bond in a 9-membered ring, both rarities in nature.
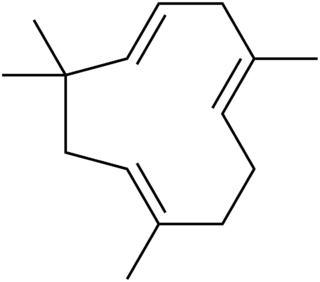
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene (C15H24), containing an 11-membered ring and consisting of 3 isoprene units containing three nonconjugated C=C double bonds, two of them being triply substituted and one being doubly substituted. It was first found in the essential oils of Humulus lupulus (hops), from which it derives its name. Humulene is an isomer of β-caryophyllene, and the two are often found together as a mixture in many aromatic plants.

Nerolidol, also known as peruviol and penetrol, is a naturally occurring sesquiterpene alcohol. A colorless liquid, it is found in the essential oils of many types of plants and flowers. There are four isomers of nerolidol', which differ in the geometry about the central double bond and configuration of the hydroxyl-bearing carbon, but most applications use such a mixture. The aroma of nerolidol is woody and reminiscent of fresh bark. It is used as a flavoring agent and in perfumery as well as in non-cosmetic products such as detergents and cleansers. Nerolidyl derivatives include nerolidyl diphosphate and the fragrance nerolidyl acetate.

Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. A recent study conducted in the Cosmics Leaving Outdoor Droplets large cloud chamber at CERN, has identified sesquiterpenes—gaseous hydrocarbons that are released by plants—as potentially playing a major role in cloud formation in relatively pristine regions of the atmosphere.

Cannabis flower essential oil, also known as hemp essential oil, is an essential oil obtained by steam distillation from the flowers, panicles, stem, and upper leaves of the hemp plant. Hemp essential oil is distinct from hemp seed oil and hash oil: the former is a vegetable oil that is cold-pressed from the seeds of low-THC varieties of hemp, the latter is a THC-rich extract of dried female hemp flowers (marijuana) or resin (hashish).
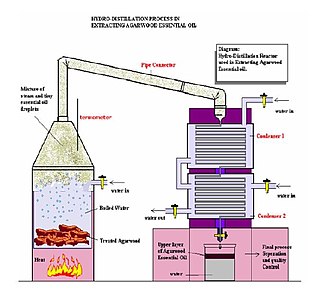
Herbal distillates, also known as floral waters, hydrosols, hydrolates, herbal waters, and essential waters, are aqueous products of hydrodistillation. They are colloidal suspensions of essential oils as well as water-soluble components obtained by steam distillation or hydrodistillation from plants and herbs. These herbal distillates have uses as flavorings and cosmetics. Common herbal distillates for skincare include rose water, orange flower water, and witch hazel. Rosemary, oregano, and thyme are hydrosols that may be used in food manufacturing industries.

Germacrenes are a class of volatile organic hydrocarbons, specifically, sesquiterpenes. Germacrenes are typically produced in a number of plant species for their antimicrobial and insecticidal properties, though they also play a role as insect pheromones. Two prominent molecules are germacrene A and germacrene D.

Aromatherapy is a practice based on the use of aromatic materials, including essential oils and other aroma compounds, with claims for improving psychological well-being. It is used as a complementary therapy or as a form of alternative medicine, and typically is used via inhalation and not by ingestion.

α-Cadinol or 10α-hydroxy-4-cadinene is an organic compound, a sesquiterpenoid alcohol.

2,5-Dimethoxy-p-cymene, or thymohydroquinone dimethyl ether, is a phytochemical found in the essential oils of plants within the family Asteraceae. These essential oils, which contain the compound as a major component of the oil, have antifungal, antibacterial, and insecticidal properties.

Viridiflorol is a chemical compound, classified as a sesquiterpenoid, that has been isolated from the essential oils of a variety of plants including Melaleuca quinquenervia, Melaleuca alternifolia, and Allophylus edulis.

The oil bodies of liverworts, occasionally dubbed complex oil bodies for distinction, are unique organelles exclusive to the Marchantiophyta. They are markedly different from the oil bodies found in other land plants and algae in that they are membrane-bound, and not associated with food storage. The organelles are variable and present in an estimated 90% of liverwort species, often proving taxonomically relevant. As a whole, the formation and function of the organelles are poorly understood. Complex oil bodies are recognized as sites of isoprenoid biosynthesis and essential oil accumulation, and have been implicated with anti-herbivory, desiccation tolerance, and photo-protection.
Turmerones are a group of related chemical compounds of the sesquiterpene class. They are found in turmeric, from which they derive their name, as well as other related plants such as Curcuma caesia. There are multiple structural types of turmerones which differ in the number and placement of double bonds including α-tumerone, β-turmerone, and ar-turmerone. Each of these types consists of multiple stereoisomers.

Mustakone is a tricylic sesquiterpenoid with the chemical formula C15H22O. It is named after the plant it was first extracted from Cyperus rotundus, which had the common name "mustuka" in Hindi. Mustakone can be found in a variety of plants and their oils like Myrcia sylvatica, Cyperus articulatus, and Hymenaea courbaril.


















