Related Research Articles

Dynamite is an explosive made of nitroglycerin, sorbents, and stabilizers. It was invented by the Swedish chemist and engineer Alfred Nobel in Geesthacht, Northern Germany, and was patented in 1867. It rapidly gained wide-scale use as a more robust alternative to the traditional black powder explosives. It allows the use of nitroglycerine's favorable explosive properties while greatly reducing its risk of accidental detonation.

An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.
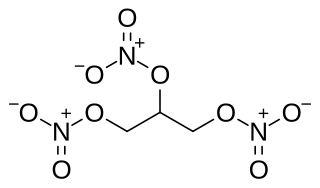
Nitroglycerin (NG), also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Discovered in 1847 by Ascanio Sobrero, nitroglycerin has been used as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. It is combined with nitrocellulose to form double-based smokeless powder, which has been used as a propellant in artillery and firearms since the 1880s.

Trinitrotoluene, more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

Cordite is a family of smokeless propellants developed and produced in Britain since 1889 to replace black powder as a military firearm propellant. Like modern gunpowder, cordite is classified as a low explosive because of its slow burning rates and consequently low brisance. These produce a subsonic deflagration wave rather than the supersonic detonation wave produced by brisants, or high explosives. The hot gases produced by burning gunpowder or cordite generate sufficient pressure to propel a bullet or shell to its target, but not so quickly as to routinely destroy the barrel of the gun.
Nitrocellulose is a highly flammable compound formed by nitrating cellulose through exposure to a mixture of nitric acid and sulfuric acid. One of its first major uses was as guncotton, a replacement for gunpowder as propellant in firearms. It was also used to replace gunpowder as a low-order explosive in mining and other applications. In the form of collodion it was also a critical component in an early photographic emulsion, the use of which revolutionized photography in the 1860s.

A detonator, sometimes called a blasting cap in the US, is a small sensitive device used to provoke a larger, more powerful but relatively insensitive secondary explosive of an explosive device used in commercial mining, excavation, demolition, etc.

A match is a tool for starting a fire. Typically, matches are made of small wooden sticks or stiff paper. One end is coated with a material that can be ignited by friction generated by striking the match against a suitable surface. Wooden matches are packaged in matchboxes, and paper matches are partially cut into rows and stapled into matchbooks. The coated end of a match, known as the match "head", consists of a bead of active ingredients and binder, often colored for easier inspection. There are two main types of matches: safety matches, which can be struck only against a specially prepared surface, and strike-anywhere matches, for which any suitably frictional surface can be used.

Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

"Screaming Jelly Babies", also known as "Growling Gummy Bears", is a classroom chemistry demonstration which is practiced in schools around the world. It is often used at open evenings to show the more engaging and entertaining aspects of science in secondary education settings.

Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used

Christian Friedrich Schönbein HFRSE was a German-Swiss chemist who is best known for inventing the fuel cell (1838) at the same time as William Robert Grove and his discoveries of guncotton and ozone.

Nitrobenzene is an aromatic nitro compound and the simplest of the nitrobenzenes, with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

Hexanitrobenzene, also known as HNB, is a nitrobenzene compound in which six nitro groups are bonded to all six positions of a central benzene ring. is a high-density explosive compound with chemical formula C6N6O12, obtained by oxidizing the amine group of pentanitroaniline with hydrogen peroxide in sulfuric acid.

A squib is a miniature explosive device used in a wide range of industries, from special effects to military applications. It resembles a tiny stick of dynamite, both in appearance and construction, but has considerably less explosive power. A squib consists of two electrical leads separated by a plug of insulating material; a small bridge wire or electrical resistance heater; and a bead of heat-sensitive chemical composition, in which the bridge wire is embedded. They can be used to generate mechanical force to shatter or propel various materials; and for pyrotechnic effects for film and live theatrics.

Smokeless powder is a type of propellant used in firearms and artillery that produces less smoke and less fouling when fired compared to black powder. Because of their similar use, both the original black powder formulation and the smokeless propellant which replaced it are commonly described as gunpowder. The combustion products of smokeless powder are mainly gaseous, compared to around 55% solid products for black powder. In addition, smokeless powder does not leave the thick, heavy fouling of hygroscopic material associated with black powder that causes rusting of the barrel.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.

Potassium picrate, or potassium 2,4,6-trinitrophenolate, is an organic chemical, a picrate of potassium. It is a reddish yellow or green crystalline material. It is a primary explosive. Anhydrous potassium picrate forms orthorhombic crystals.
Dinitrobenzenes are nitrobenzenes composed of a benzene ring and two nitro group (-NO2) substituents. The three possible arrangements of the nitro groups afford three isomers, 1,2-dinitrobenzene, 1,3-dinitrobenzene, and 1,4-dinitrobenzene. Each isomer has the chemical formula C6H4N2O4 and a molar mass of about 168.11 g/mol. 1,3-Dinitrobenzene is the most common isomer and it is used in the manufacture of explosives.

A water-gel explosive is a fuel sensitized explosive mixture consisting of an aqueous ammonium nitrate solution that acts as the oxidizer. Water gels that are cap-insensitive are referred to under United States safety regulations as blasting agents. Water gel explosives have a jelly-like consistency and come in sausage-like packing stapled shut on both sides.
References
- 1 2 Scientific American: Supplement. Vol. 27. Munn and Company. 1889.
Kinetite (the name no doubt from, "I move") was patented by T. Petrey and O. Fallenstein (their English patent is No. 10,936, August 6, 1884). As described in their specification, kinetite is made by dissolving gun cotton in nitrobenzene
- 1 2 3 4 5 Cundill, J. P.; Thomson, J. H. (1895). A dictionary of explosives (Second ed.). London: HM Stationery Office. pp. 80–81. Retrieved 4 February 2014.
- ↑ "(untitled)". Otago Daily Times . Dunedin, New Zealand: Robert Nobel Adams. 19 August 1885. p. 4. Retrieved 4 February 2014.
- 1 2 "A New Explosive". The West Australian. Perth, Western Australia: A. Davidson, for the West Australian. 10 April 1885. p. 3. Retrieved 4 February 2014.
- 1 2 "(untitled)". The Thames Advertiser. Vol. XVI, no. 5112. Thames, New Zealand. 9 March 1885. p. 3. Retrieved 4 February 2014.
- ↑ France. Commission des Substances Explosives; North of England Institute of Mining and Mechanical Engineers (1890). Report of the (French) commission on the use of explosives in the presence of fire-damp in mines ... Lambert and Co., Ltd. Retrieved 4 February 2014.
- ↑ "A monthly record for all interested in chemical manufactures". Journal of the Society of Chemical Industry. 6 (1): 1–53. 1887. doi:10.1002/jctb.5000060101. ISSN 0368-4075.
- ↑ Sanford, P. Gerald (1906). Nitro-Explosives: A Practical Treatise (Second ed.). London: Crosby Lockwood and Son. Retrieved 4 February 2014.