
Bradycardia, also called bradyarrhythmia, is a resting heart rate under 60 beats per minute (BPM). While bradycardia can result from various pathologic processes, it is commonly a physiologic response to cardiovascular conditioning or due to asymptomatic type 1 atrioventricular block.

Brugada syndrome (BrS) is a genetic disorder in which the electrical activity of the heart is abnormal due to channelopathy. It increases the risk of abnormal heart rhythms and sudden cardiac death. Those affected may have episodes of syncope. The abnormal heart rhythms seen in those with Brugada syndrome often occur at rest. They may be triggered by a fever.

Wolff–Parkinson–White syndrome (WPWS) is a disorder due to a specific type of problem with the electrical system of the heart involving an accessory pathway able to conduct electrical current between the atria and the ventricles, thus bypassing the atrioventricular node. About 60% of people with the electrical problem developed symptoms, which may include an abnormally fast heartbeat, palpitations, shortness of breath, lightheadedness, or syncope. Rarely, cardiac arrest may occur. The most common type of irregular heartbeat that occurs is known as paroxysmal supraventricular tachycardia.

Sinus node dysfunction (SND), also known as sick sinus syndrome (SSS), is a group of abnormal heart rhythms (arrhythmias) usually caused by a malfunction of the sinus node, the heart's primary pacemaker. Tachycardia-bradycardia syndrome is a variant of sick sinus syndrome in which the arrhythmia alternates between fast and slow heart rates.

The atrioventricular node or AV node electrically connects the heart's atria and ventricles to coordinate beating in the top of the heart; it is part of the electrical conduction system of the heart. The AV node lies at the lower back section of the interatrial septum near the opening of the coronary sinus, and conducts the normal electrical impulse from the atria to the ventricles. The AV node is quite compact.

Third-degree atrioventricular block is a medical condition in which the electrical impulse generated in the sinoatrial node in the atrium of the heart can not propagate to the ventricles.

The cardiac conduction system transmits the signals generated by the sinoatrial node – the heart's pacemaker, to cause the heart muscle to contract, and pump blood through the body's circulatory system. The pacemaking signal travels through the right atrium to the atrioventricular node, along the bundle of His, and through the bundle branches to Purkinje fibers in the walls of the ventricles. The Purkinje fibers transmit the signals more rapidly to stimulate contraction of the ventricles.

First-degree atrioventricular block is a disease of the electrical conduction system of the heart in which electrical impulses conduct from the cardiac atria to the ventricles through the atrioventricular node more slowly than normal. First degree AV block does not generally cause any symptoms, but may progress to more severe forms of heart block such as second- and third-degree atrioventricular block. It is diagnosed using an electrocardiogram, and is defined as a PR interval greater than 200 milliseconds. First degree AV block affects 0.65-1.1% of the population with 0.13 new cases per 1000 persons each year.

Trifascicular block is a problem with the electrical conduction of the heart, specifically the three fascicles of the bundle branches that carry electrical signals from the atrioventricular node to the ventricles. The three fascicles are one in the right bundle branch, and two in the left bundle branch the left anterior fascicle and the left posterior fascicle. A block at any of these levels can cause an abnormality to show on an electrocardiogram.

AV-nodal reentrant tachycardia (AVNRT) is a type of abnormal fast heart rhythm. It is a type of supraventricular tachycardia (SVT), meaning that it originates from a location within the heart above the bundle of His. AV nodal reentrant tachycardia is the most common regular supraventricular tachycardia. It is more common in women than men. The main symptom is palpitations. Treatment may be with specific physical maneuvers, medications, or, rarely, synchronized cardioversion. Frequent attacks may require radiofrequency ablation, in which the abnormally conducting tissue in the heart is destroyed.
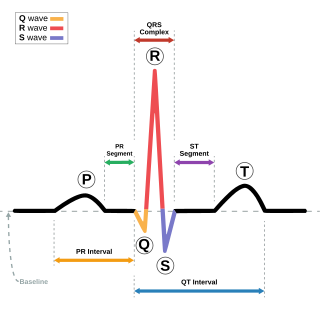
Romano–Ward syndrome is the most common form of congenital Long QT syndrome (LQTS), a genetic heart condition that affects the electrical properties of heart muscle cells. Those affected are at risk of abnormal heart rhythms which can lead to fainting, seizures, or sudden death. Romano–Ward syndrome can be distinguished clinically from other forms of inherited LQTS as it affects only the electrical properties of the heart, while other forms of LQTS can also affect other parts of the body.
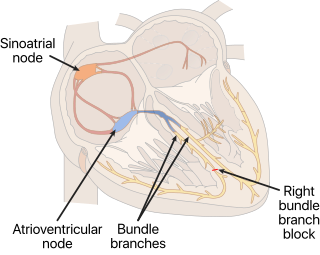
A right bundle branch block (RBBB) is a heart block in the right bundle branch of the electrical conduction system.

Atrioventricular block is a type of heart block that occurs when the electrical signal traveling from the atria, or the upper chambers of the heart, to ventricles, or the lower chambers of the heart, is impaired. Normally, the sinoatrial node produces an electrical signal to control the heart rate. The signal travels from the SA node to the ventricles through the atrioventricular node. In an AV block, this electrical signal is either delayed or completely blocked. When the signal is completely blocked, the ventricles produce their own electrical signal to control the heart rate. The heart rate produced by the ventricles is much slower than that produced by the SA node.
Sodium channel protein type 5 subunit alpha, also known as NaV1.5 is an integral membrane protein and tetrodotoxin-resistant voltage-gated sodium channel subunit. NaV1.5 is found primarily in cardiac muscle, where it mediates the fast influx of Na+-ions (INa) across the cell membrane, resulting in the fast depolarization phase of the cardiac action potential. As such, it plays a major role in impulse propagation through the heart. A vast number of cardiac diseases is associated with mutations in NaV1.5 (see paragraph genetics). SCN5A is the gene that encodes the cardiac sodium channel NaV1.5.

Junctional ectopic tachycardia (JET) is a rare syndrome of the heart that manifests in patients recovering from heart surgery. It is characterized by cardiac arrhythmia, or irregular beating of the heart, caused by abnormal conduction from or through the atrioventricular node. In newborns and infants up to 6 weeks old, the disease may also be referred to as His bundle tachycardia or congenital JET.

In electrocardiography, left axis deviation (LAD) is a condition wherein the mean electrical axis of ventricular contraction of the heart lies in a frontal plane direction between −30° and −90°. This is reflected by a QRS complex positive in lead I and negative in leads aVF and II.

Arrhythmias, also known as cardiac arrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults – is called tachycardia, and a resting heart rate that is too slow – below 60 beats per minute – is called bradycardia. Some types of arrhythmias have no symptoms. Symptoms, when present, may include palpitations or feeling a pause between heartbeats. In more serious cases, there may be lightheadedness, passing out, shortness of breath, chest pain, or decreased level of consciousness. While most cases of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in sudden death.
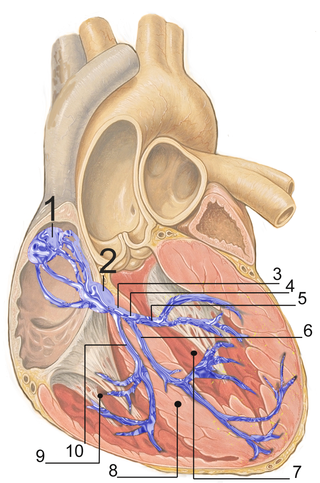
The bundle branches, or Tawara branches, are offshoots of the bundle of His in the heart's ventricle. They play an integral role in the electrical conduction system of the heart by transmitting cardiac action potentials from the bundle of His to the Purkinje fibers.

The congenital heart block (CHB) is the heart block that is diagnosed in fetus or within the first 28 days after birth, some studies also include the diagnosis during early childhood to the definition of CHB. It refers to the disorder in the electrical conduction system within the heart muscle, which leads to the failure in pumping the blood efficiently into the aorta and the pulmonary trunk. The result of CHB can be first, second, or third-degree (complete) atrioventricular block in which no electric signals move from the atrium to the ventricles



















