
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar System, but with attraction provided by electrostatic forces in place of gravity. After the solar system Joseph Larmor model (1897), the cubical model (1902), the Hantaro Nagaoka Saturnian model (1904), the plum pudding model (1904), the quantum Arthur Haas model (1910), the Rutherford model (1911), and the nuclear quantum John William Nicholson model (1912), came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement over the 1911 Rutherford model mainly concerned the new quantum physical interpretation introduced by Haas and Nicholson, but forsaking any attempt to align with classical physics radiation.

A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized.

In the physical sciences, the wavenumber is the spatial frequency of a wave, measured in cycles per unit distance or radians per unit distance. It is analogous to temporal frequency, which is defined as the number of wave cycles per unit time or radians per unit time.
In spectroscopy, the Rydberg constant, symbol for heavy atoms or for hydrogen, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to the electromagnetic spectra of an atom. The constant first arose as an empirical fitting parameter in the Rydberg formula for the hydrogen spectral series, but Niels Bohr later showed that its value could be calculated from more fundamental constants via his Bohr model. As of 2018, and electron spin g-factor are the most accurately measured physical constants.

Johannes (Janne) Robert Rydberg was a Swedish physicist mainly known for devising the Rydberg formula, in 1888, which is used to describe the wavelengths of photons emitted by changes in the energy level of an electron in a hydrogen atom.
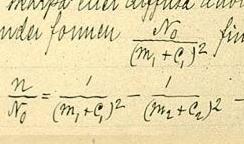
In atomic physics, the Rydberg formula calculates the wavelengths of a spectral line in many chemical elements. The formula was primarily presented as a generalization of the Balmer series for all atomic electron transitions of hydrogen. It was first empirically stated in 1888 by the Swedish physicist Johannes Rydberg, then theoretically by Niels Bohr in 1913, who used a primitive form of quantum mechanics. The formula directly generalizes the equations used to calculate the wavelengths of the hydrogen spectral series.
In physics and chemistry, the Lyman series is a hydrogen spectral series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1, the lowest energy level of the electron. The transitions are named sequentially by Greek letters: from n = 2 to n = 1 is called Lyman-alpha, 3 to 1 is Lyman-beta, 4 to 1 is Lyman-gamma, and so on. The series is named after its discoverer, Theodore Lyman. The greater the difference in the principal quantum numbers, the higher the energy of the electromagnetic emission.

Johann Jakob Balmer was a Swiss mathematician best known for his work in physics, the Balmer series of Hydrogen atom.
Rydberg may refer to:
A Rydberg atom is an excited atom with one or more electrons that have a very high principal quantum number, n. The higher the value of n, the farther the electron is from the nucleus, on average. Rydberg atoms have a number of peculiar properties including an exaggerated response to electric and magnetic fields, long decay periods and electron wavefunctions that approximate, under some conditions, classical orbits of electrons about the nuclei. The core electrons shield the outer electron from the electric field of the nucleus such that, from a distance, the electric potential looks identical to that experienced by the electron in a hydrogen atom.

The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the series by the Rydberg formula was important in the development of quantum mechanics. The spectral series are important in astronomical spectroscopy for detecting the presence of hydrogen and calculating red shifts.
The term quantum defect refers to two concepts: energy loss in lasers and energy levels in alkali elements. Both deal with quantum systems where matter interacts with light.
The Rydberg–Ritz combination principle is an empirical generalization proposed by Walther Ritz in 1908 to describe the relationship of the spectral lines for all atoms. The principle states that the spectral lines of any element include frequencies that are either the sum or the difference of the frequencies of two other lines. Lines of the spectra of elements could be predicted from existing lines. Since the frequency of light is proportional to the wavenumber or reciprocal wavelength, the principle can also be expressed in terms of wavenumbers which are the sum or difference of wavenumbers of two other lines.
The Rydberg states of an atom or molecule are electronically excited states with energies that follow the Rydberg formula as they converge on an ionic state with an ionization energy. Although the Rydberg formula was developed to describe atomic energy levels, it has been used to describe many other systems that have electronic structure roughly similar to atomic hydrogen. In general, at sufficiently high principal quantum numbers, an excited electron - ionic core system will have the general character of a hydrogenic system and the energy levels will follow the Rydberg formula. Rydberg states have energies converging on the energy of the ion. The ionization energy threshold is the energy required to completely liberate an electron from the ionic core of an atom or molecule. In practice, a Rydberg wave packet is created by a laser pulse on a hydrogenic atom and thus populates a superposition of Rydberg states. Modern investigations using pump-probe experiments show molecular pathways – e.g. dissociation of (NO)2 – via these special states.
R is the eighteenth letter of the Latin alphabet.
Quantum electrodynamics (QED), a relativistic quantum field theory of electrodynamics, is among the most stringently tested theories in physics. The most precise and specific tests of QED consist of measurements of the electromagnetic fine-structure constant, α, in various physical systems. Checking the consistency of such measurements tests the theory.
Rydberg matter is an exotic phase of matter formed by Rydberg atoms; it was predicted around 1980 by É. A. Manykin, M. I. Ozhovan and P. P. Poluéktov. It has been formed from various elements like caesium, potassium, hydrogen and nitrogen; studies have been conducted on theoretical possibilities like sodium, beryllium, magnesium and calcium. It has been suggested to be a material that diffuse interstellar bands may arise from. Circular Rydberg states, where the outermost electron is found in a planar circular orbit, are the most long-lived, with lifetimes of up to several hours, and are the most common.
The electron mass is the mass of a stationary electron, also known as the invariant mass of the electron. It is a fundamental constants of physics. It has a value of about 9.109×10−31 kilograms or about 5.486×10−4 daltons, equivalent to an energy of about 8.187×10−14 joules or about 0.5110 MeV.
The Lyman limit is the short-wavelength end of the hydrogen Lyman series, at 91.2 nm (912 Å). It corresponds to the energy required for an electron in the hydrogen ground state to escape from the electric potential barrier that originally confined it, thus creating a hydrogen ion. This energy is equivalent to the Rydberg constant.
In physics, natural units are physical units of measurement based only on universal physical constants. For example, the elementary charge e is a natural unit of electric charge, and the speed of light c is a natural unit of speed. A purely natural system of units has all of its units usually defined such that the numerical values of the selected physical constants in terms of these units are exactly 1. These constants may then be omitted from mathematical expressions of physical laws, and while this has the apparent advantage of simplicity, it may entail a loss of clarity due to the loss of information for dimensional analysis. It precludes the interpretation of an expression in terms of fundamental physical constants, such as e and c, unless it is known which units the expression is supposed to have. In this case, the reinsertion of the correct powers of e, c, etc., can be uniquely determined.