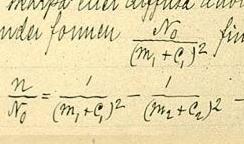History
In 1890, Rydberg proposed on a formula describing the relation between the wavelengths in spectral lines of alkali metals. [2] : v1:376 He noticed that lines came in series and he found that he could simplify his calculations by specifying the lines in terms of their wavenumber (the number of waves occupying the unit length, equal to 1/λ, the inverse of the wavelength) rather than their wavelength. He plotted the wavenumbers (n) of successive lines in each series against consecutive integers which represented the order of the lines in that particular series. Finding that the resulting curves were similarly shaped, he sought a single function which could generate all of them, when appropriate constants were inserted.
First he tried the formula:  , where n is the line's wavenumber, n0 is the series limit, m is the line's ordinal number in the series, m′ is a constant different for different series and C0 is a universal constant. This did not work very well.
, where n is the line's wavenumber, n0 is the series limit, m is the line's ordinal number in the series, m′ is a constant different for different series and C0 is a universal constant. This did not work very well.
Rydberg was trying:  when he became aware of Balmer's formula for the hydrogen spectrum
when he became aware of Balmer's formula for the hydrogen spectrum  In this equation, m is an integer and h is a constant (not to be confused with the later Planck constant).
In this equation, m is an integer and h is a constant (not to be confused with the later Planck constant).
Rydberg therefore rewrote Balmer's formula in terms of wavenumbers, as  .
.
This suggested that the Balmer formula for hydrogen might be a special case with  and
and  , where
, where  , the reciprocal of Balmer's constant (this constant h is written B in the Balmer equation article, again to avoid confusion with the Planck constant).
, the reciprocal of Balmer's constant (this constant h is written B in the Balmer equation article, again to avoid confusion with the Planck constant).
The term  was found to be a universal constant common to all elements, equal to 4/h. This constant is now known as the Rydberg constant, and m′ is known as the quantum defect.
was found to be a universal constant common to all elements, equal to 4/h. This constant is now known as the Rydberg constant, and m′ is known as the quantum defect.
As stressed by Niels Bohr, [3] expressing results in terms of wavenumber, not wavelength, was the key to Rydberg's discovery. The fundamental role of wavenumbers was also emphasized by the Rydberg-Ritz combination principle of 1908. The fundamental reason for this lies in quantum mechanics. Light's wavenumber is proportional to frequency  , and therefore also proportional to light's quantum energy E. Thus,
, and therefore also proportional to light's quantum energy E. Thus,  (in this formula the h represents the Planck constant). Modern and legitimate understanding is that Rydberg's findings were a reflection of the underlying simplicity of the behavior of spectral lines, in terms of fixed (quantized) energy differences between electron orbitals in atoms. Rydberg's 1888 classical expression for the form of the spectral series was not accompanied by a physical explanation. Walther Ritz's pre-quantum 1908 explanation for the mechanism underlying the spectral series was that atomic electrons behaved like magnets and that the magnets could vibrate with respect to the atomic nucleus (at least temporarily) to produce electromagnetic radiation, [4] [5] but this theory was superseded in 1913 by Niels Bohr's model of the atom.
(in this formula the h represents the Planck constant). Modern and legitimate understanding is that Rydberg's findings were a reflection of the underlying simplicity of the behavior of spectral lines, in terms of fixed (quantized) energy differences between electron orbitals in atoms. Rydberg's 1888 classical expression for the form of the spectral series was not accompanied by a physical explanation. Walther Ritz's pre-quantum 1908 explanation for the mechanism underlying the spectral series was that atomic electrons behaved like magnets and that the magnets could vibrate with respect to the atomic nucleus (at least temporarily) to produce electromagnetic radiation, [4] [5] but this theory was superseded in 1913 by Niels Bohr's model of the atom.
Bohr's interpretation and derivation of the constant
Rydberg's published formula was [1]  where
where  is the observed wavenumber,
is the observed wavenumber,  is a constant for all spectral series and elements, and the remaining values,
is a constant for all spectral series and elements, and the remaining values,  are integers indexing the various lines. When Bohr analyzes his model for the atom he writes [6]
are integers indexing the various lines. When Bohr analyzes his model for the atom he writes [6]  where he uses frequency
where he uses frequency  (proportional to wavenumber). Thus he has been able to compute the value of Rydberg's heuristic constant
(proportional to wavenumber). Thus he has been able to compute the value of Rydberg's heuristic constant  from his atom theory and set the integers
from his atom theory and set the integers  and
and  to zero. The effect is to predict new series corresponding to
to zero. The effect is to predict new series corresponding to  in the extreme ultraviolet unknown to Rydberg. [4]
in the extreme ultraviolet unknown to Rydberg. [4]
In Bohr's conception of the atom, the integer Rydberg (and Balmer) n numbers represent electron orbitals at different integral distances from the atom. A frequency (or spectral energy) emitted in a transition from n1 to n2 therefore represents the photon energy emitted or absorbed when an electron makes a jump from orbital 1 to orbital 2. [3]
Later models found that the values for n1 and n2 corresponded to the principal quantum numbers of the two orbitals.
The Rydberg formula can be interpreted both through the semi-classical Bohr model and through fully quantum-mechanical treatments of the hydrogen atom. In the Bohr model, electrons occupy quantized orbits whose energies vary. When an electron transitions from a higher level to a lower level a photon is emitted with a wavelength matching the Rydberg expression. [7] Modern quantum mechanics arrives at the same result from the Schrödinger equation for an electron bound by a Coulomb potential. Differences in the energy eigenvalues of the hydrogen atom reproduce the observed Rydberg dependence, while relativistic and spin corrections appear when the Dirac equation, fine-structure interactions, and quantum electrodynamics (QED) effects are included. These refinements explain small deviations from the simple formula, such as the Lamb shift and hyperfine splittings in hydrogen-like systems. [8] [7]
For any hydrogen-like element
The formula above can be extended for use with any hydrogen-like chemical elements with  where
where
This formula can be directly applied only to hydrogen-like, also called hydrogenic atoms of chemical elements, i.e. atoms with only one electron being affected by an effective nuclear charge (which is easily estimated). Examples would include He+, Li2+, Be3+ etc., where no other electrons exist in the atom [9] .
But the Rydberg formula also provides correct wavelengths for distant electrons, where the effective nuclear charge can be estimated as the same as that for hydrogen, since all but one of the nuclear charges have been screened by other electrons, and the core of the atom has an effective positive charge of +1. [10]
Finally, with certain modifications (replacement of Z by Z − 1, and use of the integers 1 and 2 for the ns to give a numerical value of 3⁄4 for the difference of their inverse squares), the Rydberg formula provides correct values in the special case of K-alpha lines, since the transition in question is the K-alpha transition of the electron from the 1s orbital to the 2p orbital. This is analogous to the Lyman-alpha line transition for hydrogen, and has the same frequency factor. [11] Because the 2p electron is not screened by any other electrons in the atom from the nucleus, the nuclear charge is diminished only by the single remaining 1s electron, causing the system to be effectively a hydrogenic atom, but with a diminished nuclear charge Z − 1. Its frequency is thus the Lyman-alpha hydrogen frequency, increased by a factor of (Z − 1)2. This formula of f = c / λ = (Lyman-alpha frequency) ⋅ (Z − 1)2 is historically known as Moseley's law (having added a factor c to convert wavelength to frequency), and can be used to predict wavelengths of the Kα (K-alpha) X-ray spectral emission lines of chemical elements from aluminum to gold. [11] See the biography of Henry Moseley for the historical importance of this law, which was derived empirically at about the same time it was explained by the Bohr model of the atom. [12]
For other spectral transitions in multi-electron atoms, the Rydberg formula generally provides incorrect results, since the magnitude of the screening of inner electrons for outer-electron transitions is variable and cannot be compensated for in the simple manner above. The correction to the Rydberg formula for these atoms is known as the quantum defect.