Related Research Articles

An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of excitable cells, which include animal cells like neurons and muscle cells, as well as some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.
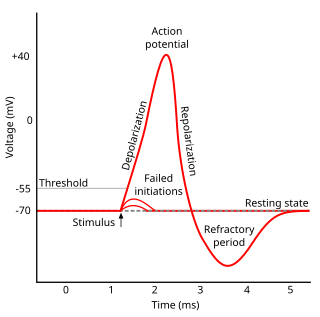
Refractoriness is the fundamental property of any object of autowave nature not responding to stimuli, if the object stays in the specific refractory state. In common sense, refractory period is the characteristic recovery time, a period that is associated with the motion of the image point on the left branch of the isocline .
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell-to-cell signalling. EPSPs and IPSPs compete with each other at numerous synapses of a neuron. This determines whether an action potential occurring at the presynaptic terminal produces an action potential at the postsynaptic membrane. Some common neurotransmitters involved in IPSPs are GABA and glycine.

Hyperpolarization is a change in a cell's membrane potential that makes it more negative. Cells typically have a negative resting potential, with neuronal action potentials depolarizing the membrane. When the resting membrane potential is made more negative, it increases the minimum stimulus needed to surpass the needed threshold. Neurons naturally become hyperpolarized at the end of an action potential, which is often referred to as the relative refractory period. Relative refractory periods typically last 2 milliseconds, during which a stronger stimulus is needed to trigger another action potential. Cells can also become hyperpolarized depending on channels and receptors present on the membrane, which can have an inhibitory effect.

In electrophysiology, the threshold potential is the critical level to which a membrane potential must be depolarized to initiate an action potential. In neuroscience, threshold potentials are necessary to regulate and propagate signaling in both the central nervous system (CNS) and the peripheral nervous system (PNS).

Unlike the action potential in skeletal muscle cells, the cardiac action potential is not initiated by nervous activity. Instead, it arises from a group of specialized cells known as pacemaker cells, that have automatic action potential generation capability. In healthy hearts, these cells form the cardiac pacemaker and are found in the sinoatrial node in the right atrium. They produce roughly 60–100 action potentials every minute. The action potential passes along the cell membrane causing the cell to contract, therefore the activity of the sinoatrial node results in a resting heart rate of roughly 60–100 beats per minute. All cardiac muscle cells are electrically linked to one another, by intercalated discs which allow the action potential to pass from one cell to the next. This means that all atrial cells can contract together, and then all ventricular cells.
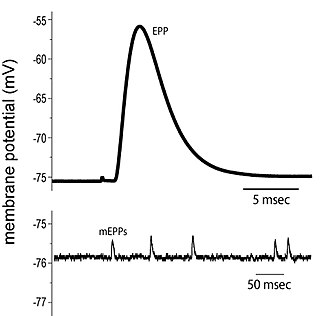
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.

In neuroscience, repolarization refers to the change in membrane potential that returns it to a negative value just after the depolarization phase of an action potential which has changed the membrane potential to a positive value. The repolarization phase usually returns the membrane potential back to the resting membrane potential. The efflux of potassium (K+) ions results in the falling phase of an action potential. The ions pass through the selectivity filter of the K+ channel pore.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g. muscle, glial cells, neurons) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+–Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions.
Visual phototransduction is the sensory transduction process of the visual system by which light is detected by photoreceptor cells in the vertebrate retina. A photon is absorbed by a retinal chromophore, which initiates a signal cascade through several intermediate cells, then through the retinal ganglion cells (RGCs) comprising the optic nerve.
T-type calcium channels are low voltage activated calcium channels that become inactivated during cell membrane hyperpolarization but then open to depolarization. The entry of calcium into various cells has many different physiological responses associated with it. Within cardiac muscle cell and smooth muscle cells voltage-gated calcium channel activation initiates contraction directly by allowing the cytosolic concentration to increase. Not only are T-type calcium channels known to be present within cardiac and smooth muscle, but they also are present in many neuronal cells within the central nervous system. Different experimental studies within the 1970s allowed for the distinction of T-type calcium channels from the already well-known L-type calcium channels. The new T-type channels were much different from the L-type calcium channels due to their ability to be activated by more negative membrane potentials, had small single channel conductance, and also were unresponsive to calcium antagonist drugs that were present. These distinct calcium channels are generally located within the brain, peripheral nervous system, heart, smooth muscle, bone, and endocrine system.
The P-type calcium channel is a type of voltage-dependent calcium channel. Similar to many other high-voltage-gated calcium channels, the α1 subunit determines most of the channel's properties. The 'P' signifies cerebellar Purkinje cells, referring to the channel's initial site of discovery. P-type calcium channels play a similar role to the N-type calcium channel in neurotransmitter release at the presynaptic terminal and in neuronal integration in many neuronal types.
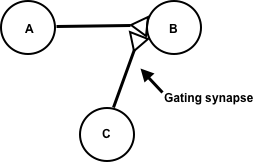
Synaptic gating is the ability of neural circuits to gate inputs by either suppressing or facilitating specific synaptic activity. Selective inhibition of certain synapses has been studied thoroughly, and recent studies have supported the existence of permissively gated synaptic transmission. In general, synaptic gating involves a mechanism of central control over neuronal output. It includes a sort of gatekeeper neuron, which has the ability to influence transmission of information to selected targets independently of the parts of the synapse upon which it exerts its action.
Neural backpropagation is the phenomenon in which, after the action potential of a neuron creates a voltage spike down the axon, another impulse is generated from the soma and propagates towards the apical portions of the dendritic arbor or dendrites. In addition to active backpropagation of the action potential, there is also passive electrotonic spread. While there is ample evidence to prove the existence of backpropagating action potentials, the function of such action potentials and the extent to which they invade the most distal dendrites remain highly controversial.

Subthreshold membrane potential oscillations are membrane oscillations that do not directly trigger an action potential since they do not reach the necessary threshold for firing. However, they may facilitate sensory signal processing.
Recurrent thalamo-cortical resonance or Thalamocortical oscillation is an observed phenomenon of oscillatory neural activity between the thalamus and various cortical regions of the brain. It is proposed by Rodolfo Llinas and others as a theory for the integration of sensory information into the whole of perception in the brain. Thalamocortical oscillation is proposed to be a mechanism of synchronization between different cortical regions of the brain, a process known as temporal binding. This is possible through the existence of thalamocortical networks, groupings of thalamic and cortical cells that exhibit oscillatory properties.

Spike-and-wave is a pattern of the electroencephalogram (EEG) typically observed during epileptic seizures. A spike-and-wave discharge is a regular, symmetrical, generalized EEG pattern seen particularly during absence epilepsy, also known as ‘petit mal’ epilepsy. The basic mechanisms underlying these patterns are complex and involve part of the cerebral cortex, the thalamocortical network, and intrinsic neuronal mechanisms.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.
In neurology, a paroxysmal depolarizing shift (PDS) or depolarizing shift is a hallmark of cellular manifestation of epilepsy. Little is known about the initiation, propagation and termination of PDS. Previously, electrophysiological studies have provided the evidence that there is a Ca2+-mediated depolarization, which causes voltage-gated Na+ channels to open, resulting in action potentials. This depolarization is followed by a period of hyperpolarization mediated by Ca2+-dependent K+ channels or GABA-activated Cl− influx.. In general, synaptic PDS could be initiated by EPSPs, and the plateau potential of the PDS is maintained by a combination of synaptic potentials (EPSPs, IPSPs) and ionic conductances (persistent sodium current and high-threshold calcium current) and the post-PDS hyperpolarization is governed by multiple potassium currents, activated by calcium or sodium entry, as well as by leak current. The next cycle of depolarization is initiated by both synaptic drive and the hyperpolarization-activated IH current.
References
- ↑ Beatty, J. A.; Sullivan, M. A.; Morikawa, H.; Wilson, C. J. (2012). "Complex autonomous firing patterns of striatal low-threshold spike interneurons". Journal of Neurophysiology. 108 (3): 771–81. doi:10.1152/jn.00283.2012. PMC 3424086 . PMID 22572945.
- ↑ Llinás, R.; Yarom, Y. (June 1981). "Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances". The Journal of Physiology. 315: 549–567. doi:10.1113/jphysiol.1981.sp013763. ISSN 0022-3751. PMC 1249398 . PMID 6273544.
- ↑ Llinás, R.; Yarom, Y. (June 1981). "Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro". The Journal of Physiology. 315: 569–584. doi:10.1113/jphysiol.1981.sp013764. ISSN 0022-3751. PMC 1249399 . PMID 7310722.
- ↑ Gutnick, MJ; Yarom, Y (1989). "Low threshold calcium spikes, intrinsic neuronal oscillation and rhythm generation in the CNS". Journal of Neuroscience Methods. 28 (1–2): 93–9. doi:10.1016/0165-0270(89)90014-9. PMID 2657227.
- ↑ Llinas, R. R.; Steriade, M (2006). "Bursting of Thalamic Neurons and States of Vigilance". Journal of Neurophysiology. 95 (6): 3297–308. doi:10.1152/jn.00166.2006. PMID 16554502. S2CID 14047398.
- ↑ Gutierrez, Carolina; Cox, Charles L.; Rinzel, John; Sherman, S. Murray (February 1, 2001). "Dynamics of Low-Threshold Spike Activation in Relay Neurons of the Cat Lateral Geniculate Nucleus". The Journal of Neuroscience. 21 (3): 1022–32. doi:10.1523/jneurosci.21-03-01022.2001. PMC 6762305 . PMID 11157087.
- ↑ Perez-Reyes, Edward (2003). "Molecular Physiology of Low-Voltage-Activated T-type Calcium Channels". Physiological Reviews. 83 (1): 117–61. doi:10.1152/physrev.00018.2002. PMID 12506128.
- ↑ Lu, SM; Guido, W; Sherman, SM (Dec 1992). "Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance". Journal of Neurophysiology. 68 (6): 2185–98. doi:10.1152/jn.1992.68.6.2185. PMID 1337104.
- ↑ Zhan, XJ; Cox, CL; Sherman, SM (2000). "Dendritic depolarization efficiently attenuates low-threshold calcium spikes in thalamic relay cells". The Journal of Neuroscience. 20 (10): 3909–14. doi:10.1523/JNEUROSCI.20-10-03909.2000. PMC 6772701 . PMID 10804230.
- 1 2 3 Zhan, XJ; Cox, CL; Rinzel, J; Sherman, SM (1999). "Current clamp and modeling studies of low-threshold calcium spikes in cells of the cat's lateral geniculate nucleus". Journal of Neurophysiology. 81 (5): 2360–73. doi:10.1152/jn.1999.81.5.2360. PMID 10322072.
- ↑ Cains, Sarah; Blomeley, Craig P.; Bracci, Enrico (2012). "Serotonin inhibits low-threshold spike interneurons in the striatum". The Journal of Physiology. 590 (10): 2241–52. doi:10.1113/jphysiol.2011.219469. PMC 3424750 . PMID 22495583.
- ↑ Siwek, Magdalena; Henseler, Christina; Broich, Karl; Papazoglou, Anna; Weiergräber, Marco (2012). "Voltage-Gated Ca2+ Channel Mediated Ca2+ Influx in Epileptogenesis". In Islam, Md. Shahidul (ed.). Calcium Signaling. Advances in Experimental Medicine and Biology. Vol. 740. pp. 1219–47. doi:10.1007/978-94-007-2888-2_55. ISBN 978-94-007-2887-5. PMID 22453990.
- ↑ Ochoa, J; Willise, R (2012). "Antiepileptic Drugs". Medscape Reference.
- ↑ Jeanmonod, D.; Magnin, M.; Morel, A. (1996). "Low-threshold calcium spike bursts in the human thalamus". Brain. 119 (2): 363–75. doi: 10.1093/brain/119.2.363 . PMID 8800933.
- ↑ Magnin, M; Morel, A; Jeanmonod, D (2000). "Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients". Neuroscience. 96 (3): 549–64. doi:10.1016/S0306-4522(99)00583-7. PMID 10717435.