Cerebrospinal fluid (CSF) is a clear, colorless body fluid found within the tissue that surrounds the brain and spinal cord of all vertebrates.

The blood–brain barrier (BBB) is a highly selective semipermeable border of endothelial cells that prevents solutes in the circulating blood from non-selectively crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.

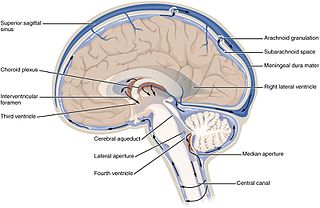
The ventricular system is a set of four interconnected cavities known as cerebral ventricles in the brain. Within each ventricle is a region of choroid plexus which produces the circulating cerebrospinal fluid (CSF). The ventricular system is continuous with the central canal of the spinal cord from the fourth ventricle, allowing for the flow of CSF to circulate.
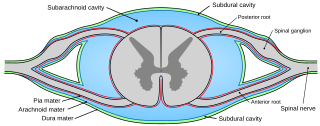
Pia mater, often referred to as simply the pia, is the delicate innermost layer of the meninges, the membranes surrounding the brain and spinal cord. Pia mater is medieval Latin meaning "tender mother". The other two meningeal membranes are the dura mater and the arachnoid mater. Both the pia and arachnoid mater are derivatives of the neural crest while the dura is derived from embryonic mesoderm. The pia mater is a thin fibrous tissue that is permeable to water and small solutes. The pia mater allows blood vessels to pass through and nourish the brain. The perivascular space between blood vessels and pia mater is proposed to be part of a pseudolymphatic system for the brain. When the pia mater becomes irritated and inflamed the result is meningitis.

The third ventricle is one of the four connected ventricles of the ventricular system within the mammalian brain. It is a slit-like cavity formed in the diencephalon between the two thalami, in the midline between the right and left lateral ventricles, and is filled with cerebrospinal fluid (CSF).
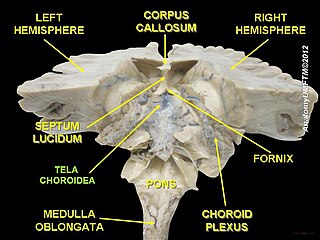
The choroid plexus, or plica choroidea, is a plexus of cells that arises from the tela choroidea in each of the ventricles of the brain. The choroid plexus produces most of the cerebrospinal fluid (CSF) of the central nervous system. CSF is produced and secreted by the regions of the choroid plexus. The choroid plexus consists of modified ependymal cells surrounding a core of capillaries and loose connective tissue.

The ependyma is the thin neuroepithelial lining of the ventricular system of the brain and the central canal of the spinal cord. The ependyma is one of the four types of neuroglia in the central nervous system (CNS). It is involved in the production of cerebrospinal fluid (CSF), and is shown to serve as a reservoir for neuroregeneration.

Choroid plexus cysts (CPCs) are cysts that occur within choroid plexus of the brain. They are the most common type of intraventricular cyst. The brain contains pockets or spaces called ventricles with a spongy layer of cells and blood vessels called the choroid plexus. This is in the middle of the fetal brain. The choroid plexus has the important function of producing cerebrospinal fluid. The fluid produced by the cells of the choroid plexus fills the ventricles and then flows around the brain and the spinal cord to provide a cushion of fluid around these structures.
The median aperture drains cerebrospinal fluid (CSF) from the fourth ventricle into the cisterna magna. The two other openings of the fourth ventricle are the lateral apertures, one on the left and one on the right, which drain cerebrospinal fluid into the cerebellopontine angle cistern. The median foramen on axial images is posterior to the pons and anterior to the caudal cerebellum. It is surrounded by the obex and gracile tubercles of the medulla, tela choroidea of the fourth ventricle and its choroid plexus, which is attached to the cerebellar vermis.

In the brain, the interventricular foramina are channels that connect the paired lateral ventricles with the third ventricle at the midline of the brain. As channels, they allow cerebrospinal fluid (CSF) produced in the lateral ventricles to reach the third ventricle and then the rest of the brain's ventricular system. The walls of the interventricular foramina also contain choroid plexus, a specialized CSF-producing structure, that is continuous with that of the lateral and third ventricles above and below it.
Circumventricular organs (CVOs) are structures in the brain characterized by their extensive and highly permeable capillaries, unlike those in the rest of the brain where there exists a blood–brain barrier (BBB) at the capillary level. Although the term "circumventricular organs" was originally proposed in 1958 by Austrian anatomist Helmut O. Hofer concerning structures around the brain ventricular system, the penetration of blood-borne dyes into small specific CVO regions was discovered in the early 20th century. The permeable CVOs enabling rapid neurohumoral exchange include the subfornical organ (SFO), the area postrema (AP), the vascular organ of lamina terminalis, the median eminence, the pituitary neural lobe, and the pineal gland.

The tela choroidea is a region of meningeal pia mater that adheres to the underlying ependyma, and gives rise to the choroid plexus in each of the brain’s four ventricles. Tela is Latin for woven and is used to describe a web-like membrane or layer. The tela choroidea is a very thin part of the loose connective tissue of pia mater overlying and closely adhering to the ependyma. It has a rich blood supply. The ependyma and vascular pia mater – the tela choroidea, form regions of minute projections known as a choroid plexus that projects into each ventricle. The choroid plexus produces most of the cerebrospinal fluid of the central nervous system that circulates through the ventricles of the brain, the central canal of the spinal cord, and the subarachnoid space. The tela choroidea in the ventricles forms from different parts of the roof plate in the development of the embryo.
The subependymal zone (SEZ) is a cell layer below the ependyma in the lateral ventricles of the brain. It is an adult version of the embryonic forebrain germinal zone. This region contains adult neural stem cells, also called neuroepithelial cells, which have the potential to generate new neurons and glial cells. The generation of neurons and glial cells from neuroepithelial cells occurs via neurogenesis and gliogenesis, respectively. In adults, the subependymal zone is also called the subventricular zone, as the ependymal cell layer forms the boundary between the fluid-filled ventricular space and the walls of the lateral ventricles.
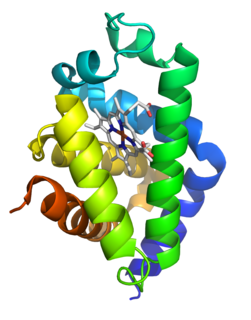
Neuroglobin is a member of the vertebrate globin family involved in cellular oxygen homeostasis and reactive oxygen/nitrogen scavenging. It is an intracellular hemoprotein expressed in the central and peripheral nervous system, cerebrospinal fluid, retina and endocrine tissues. Neuroglobin is a monomer that reversibly binds oxygen with an affinity higher than that of hemoglobin. It also increases oxygen availability to brain tissue and provides protection under hypoxic or ischemic conditions, potentially limiting brain damage. Neuroglobin were in the past found only in vertebrate neurons, but recently in 2013, were found in the neurons of unrelated protostomes, like photosynthetic acoel as well as radiata such as jelly fish. In addition to neurons, neuroglobin is present in astrocytes in certain pathologies of the rodent brain and in the physiological seal brain. This is thought to be due to convergent evolution. It is of ancient evolutionary origin, and is homologous to nerve globins of invertebrates. Recent research confirmed the presence of human neuroglobin protein in cerebrospinal fluid (CSF).

A choroid plexus carcinoma is a type of choroid plexus tumor that affects the choroid plexus of the brain. It is considered the worst of the three grades of chord plexus tumors, having a much poorer prognosis than choroid atypical plexus papilloma and choroid plexus papilloma. The disease creates lesions in the brain and increases cerebrospinal fluid volume, resulting in hydrocephalus.

The glymphatic system was described and named in 2013 as a system for waste clearance in the central nervous system (CNS) of vertebrates. According to this model, cerebrospinal fluid (CSF) flows into the paravascular space around cerebral arteries, combining with interstitial fluid (ISF) and parenchymal solutes, and exiting down venous paravascular spaces. The pathway consists of a para-arterial influx route for CSF to enter the brain parenchyma, coupled to a clearance mechanism for the removal of interstitial fluid (ISF) and extracellular solutes from the interstitial compartments of the brain and spinal cord. Exchange of solutes between CSF and ISF is driven primarily by arterial pulsation and regulated during sleep by the expansion and contraction of brain extracellular space. Clearance of soluble proteins, waste products, and excess extracellular fluid is accomplished through convective bulk flow of ISF, facilitated by astrocytic aquaporin 4 (AQP4) water channels.

Hereditary folate malabsorption (HFM) is a rare autosomal recessive disorder caused by loss-of-function mutations in the proton-coupled folate transporter (PCFT) gene, resulting in systemic folate deficiency and impaired delivery of folate to the brain.
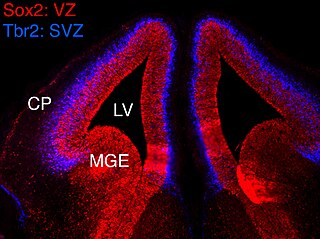
In vertebrates, the ventricular zone (VZ) is a transient embryonic layer of tissue containing neural stem cells, principally radial glial cells, of the central nervous system (CNS). The VZ is so named because it lines the ventricular system, which contains cerebrospinal fluid (CSF). The embryonic ventricular system contains growth factors and other nutrients needed for the proper function of neural stem cells. Neurogenesis, or the generation of neurons, occurs in the VZ during embryonic and fetal development as a function of the Notch pathway, and the newborn neurons must migrate substantial distances to their final destination in the developing brain or spinal cord where they will establish neural circuits. A secondary proliferative zone, the subventricular zone (SVZ), lies adjacent to the VZ. In the embryonic cerebral cortex, the SVZ contains intermediate neuronal progenitors that continue to divide into post-mitotic neurons. Through the process of neurogenesis, the parent neural stem cell pool is depleted and the VZ disappears. The balance between the rates of stem cell proliferation and neurogenesis changes during development, and species from mouse to human show large differences in the number of cell cycles, cell cycle length, and other parameters, which is thought to give rise to the large diversity in brain size and structure.

Claire Julie Liliane Wyart is a French neuroscientist and biophysicist, studying the circuits underlying the control of locomotion. She is a chevalier of the Ordre national du Mérite.
















