Related Research Articles

Edward Mills Purcell was an American physicist who shared the 1952 Nobel Prize for Physics for his independent discovery of nuclear magnetic resonance in liquids and in solids. Nuclear magnetic resonance (NMR) has become widely used to study the molecular structure of pure materials and the composition of mixtures. Friends and colleagues knew him as Ed Purcell.
Dynamic nuclear polarization (DNP) results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann distribution under the thermal equilibrium. It is also possible that those electrons are aligned to a higher degree of order by other preparations of electron spin order such as: chemical reactions, optical pumping and spin injection. DNP is considered one of several techniques for hyperpolarization. DNP can also be induced using unpaired electrons produced by radiation damage in solids.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. This spectroscopy is based on the measurement of absorption of electromagnetic radiations in the radio frequency region from roughly 4 to 900 MHz. Absorption of radio waves in the presence of magnetic field is accompanied by a special type of nuclear transition, and for this reason, such type of spectroscopy is known as Nuclear Magnetic Resonance Spectroscopy. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.
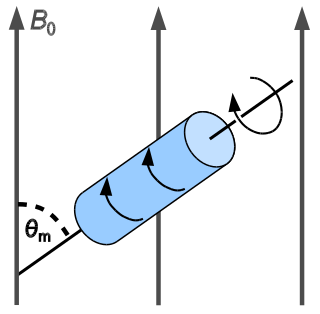
In solid-state NMR spectroscopy, magic-angle spinning (MAS) is a technique routinely used to produce better resolution NMR spectra. MAS NMR consists in spinning the sample at the magic angle θm with respect to the direction of the magnetic field.

Anatole Abragam was a French physicist who wrote The Principles of Nuclear Magnetism and made significant contributions to the field of nuclear magnetic resonance. Originally from Griva, Courland Governorate, Russian Empire, Abragam and his family emigrated to France in 1925.
In MRI and NMR spectroscopy, an observable nuclear spin polarization (magnetization) is created by a homogeneous magnetic field. This field makes the magnetic dipole moments of the sample precess at the resonance (Larmor) frequency of the nuclei. At thermal equilibrium, nuclear spins precess randomly about the direction of the applied field. They become abruptly phase coherent when they are hit by radiofrequent (RF) pulses at the resonant frequency, created orthogonal to the field. The RF pulses cause the population of spin-states to be perturbed from their thermal equilibrium value. The generated transverse magnetization can then induce a signal in an RF coil that can be detected and amplified by an RF receiver. The return of the longitudinal component of the magnetization to its equilibrium value is termed spin-latticerelaxation while the loss of phase-coherence of the spins is termed spin-spin relaxation, which is manifest as an observed free induction decay (FID).

Robert Blinc was a prominent Slovene physicist a full professor of physics and, with more than 650 articles in prestigious international journals and two extensive monographs published abroad, a highly regarded and quoted researcher in condensed matter physics.

Nuclear magnetic resonance quantum computing (NMRQC) is one of the several proposed approaches for constructing a quantum computer, that uses the spin states of nuclei within molecules as qubits. The quantum states are probed through the nuclear magnetic resonances, allowing the system to be implemented as a variation of nuclear magnetic resonance spectroscopy. NMR differs from other implementations of quantum computers in that it uses an ensemble of systems, in this case molecules, rather than a single pure state.
In nuclear chemistry and nuclear physics, J-couplings are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy, J-coupling contains information about relative bond distances and angles. Most importantly, J-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.

In physics, the spin–spin relaxation is the mechanism by which Mxy, the transverse component of the magnetization vector, exponentially decays towards its equilibrium value in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI). It is characterized by the spin–spin relaxation time, known as T2, a time constant characterizing the signal decay. It is named in contrast to T1, the spin–lattice relaxation time. It is the time it takes for the magnetic resonance signal to irreversibly decay to 37% (1/e) of its initial value after its generation by tipping the longitudinal magnetization towards the magnetic transverse plane. Hence the relation
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.
Low field NMR spans a range of different nuclear magnetic resonance (NMR) modalities, going from NMR conducted in permanent magnets, supporting magnetic fields of a few tesla (T), all the way down to zero field NMR, where the Earth's field is carefully shielded such that magnetic fields of nanotesla (nT) are achieved where nuclear spin precession is close to zero. In a broad sense, Low-field NMR is the branch of NMR that is not conducted in superconducting high-field magnets. Low field NMR also includes Earth's field NMR where simply the Earth's magnetic field is exploited to cause nuclear spin-precession which is detected. With magnetic fields on the order of μT and below magnetometers such as SQUIDs or atomic magnetometers are used as detectors. "Normal" high field NMR relies on the detection of spin-precession with inductive detection with a simple coil. However, this detection modality becomes less sensitive as the magnetic field and the associated frequencies decrease. Hence the push toward alternative detection methods at very low fields.
Herbert Sander Gutowsky was an American chemist who was a professor of chemistry at the University of Illinois Urbana-Champaign. Gutowsky was the first to apply nuclear magnetic resonance (NMR) methods to the field of chemistry. He used nuclear magnetic resonance spectroscopy to determine the structure of molecules. His pioneering work developed experimental control of NMR as a scientific instrument, connected experimental observations with theoretical models, and made NMR one of the most effective analytical tools for analysis of molecular structure and dynamics in liquids, solids, and gases, used in chemical and medical research, His work was relevant to the solving of problems in chemistry, biochemistry, and materials science, and has influenced many of the subfields of more recent NMR spectroscopy.
Raymond Freeman FRS was a British chemist and professor at Jesus College, Cambridge who made important contributions to NMR spectroscopy.
Nuclear magnetic resonance (NMR) in porous materials covers the application of using NMR as a tool to study the structure of porous media and various processes occurring in them. This technique allows the determination of characteristics such as the porosity and pore size distribution, the permeability, the water saturation, the wettability, etc.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
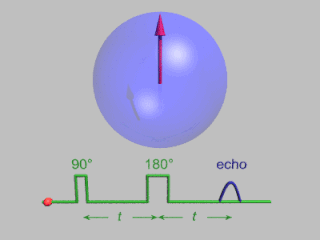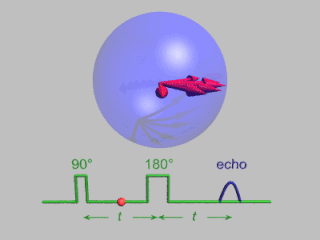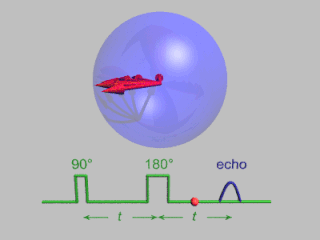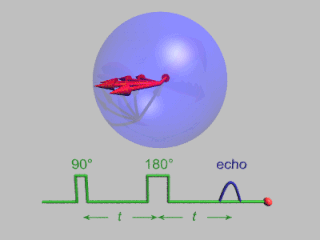
Pulsed electron paramagnetic resonance (EPR) is an electron paramagnetic resonance technique that involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short oscillating field, usually a microwave pulse. One can then measure the emitted microwave signal which is created by the sample magnetization. Fourier transformation of the microwave signal yields an EPR spectrum in the frequency domain. With a vast variety of pulse sequences it is possible to gain extensive knowledge on structural and dynamical properties of paramagnetic compounds. Pulsed EPR techniques such as electron spin echo envelope modulation (ESEEM) or pulsed electron nuclear double resonance (ENDOR) can reveal the interactions of the electron spin with its surrounding nuclear spins.

Jean Louis Charles Jeener is a Belgian physical chemist and physicist, well known for his experimental and theoretical contributions to spin thermodynamics in solids and for his invention of Two-dimensional nuclear magnetic resonance spectroscopy. He was born in Brussels in 1931, son of Raymond Jeener (biologist) and Hélène Massar. He is married to Françoise Henin.

David Lyndon Emsley FRSC is a British chemist specialising in solid-state nuclear magnetic resonance and a professor at EPFL. He was awarded the 2012 Grand Prix Charles-Leopold Mayer of the French Académie des Sciences and the 2015 Bourke Award of the Royal Society of Chemistry.
William P. Halperin is a Canadian-American physicist, academic, and researcher. He is the Orrington Lunt Professor of Physics at Northwestern University.
References
- ↑ Membre de l'Académie des Sciences Archived 2009-02-02 at the Wayback Machine , http://www.academie-sciences.fr/academie/membre/GoldmanM_bio1009.pdf%5B%5D
- ↑ Ingénieurs de la 70e promotion de l'ESPCI
- ↑ Goldman M., Landesman A., « Polarisation dynamique nucléaire par contact thermique entre des systèmes de spins », C.R. Acad. Sci., 252, (1961), p. 263-265
- ↑ Chapellier M., et al., « Production et observation d'un état antiferromagnétique nucléaire », C.R. Acad. Sci. B, 268, (1969), p. 1530-1533
- ↑ Goldman M., « Nuclear dipolar magnetic ordering », Phys. Rep., 32c, (1977), p. 1-67
- ↑ Urbina C.,et al., « Rotating transverse helical nuclear magnetic ordering », Phys. Rev. Lett., 48, (1982), p. 206-209
- ↑ Abragam A., et al., « Première observation d'une structure antiferromagnétique nucléaire par diffraction neutronique », C.R. Acad. Sci., b 286, (1978), p. 311-314
- ↑ Desvaux H. and Goldman M., « A new NMR method for measuring the rotational correlation time of molecules in the liquid state », Mol. Phys., 81, (1994), p. 955-974
- ↑ Goldman M. and Shen L., « Spin-spin relaxation in LaF3 », Phys. Rev., 144, (1966), p. 321-331
- ↑ Tabti T., et al., « Relaxation without spin diffusion in fractal systems: polymers in glassy solutions », J. Chem. Phys., 107, (1997), p. 9239-9251
- ↑ Goldman M., « Theory of EPR on a rotating sample: An illustration of Berry's phase », Eur. Phys. J., b 2, (1998), p. 147-156
- ↑ Goldman M., « Formal theory of spin-lattice relaxation », J. Magn. Reson., 149, (2001), p. 160-187
- ↑ Récipiendaires du Prix Holweck
- ↑ Quantum Description of High-Resolution NMR in Liquids sur Google Livres