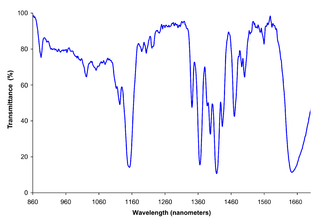
McPherson is a custom manufacturer of precision optical instruments and systems for measuring and characterizing spectra. McPherson instruments measure intensity vs. frequency in various regions of the electromagnetic spectrum. McPherson’s spectral test instruments are based on the dispersing properties of a diffraction grating and/or refractive prism.

Optics is the branch of physics that studies the behaviour and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behaviour of visible, ultraviolet, and infrared light. Because light is an electromagnetic wave, other forms of electromagnetic radiation such as X-rays, microwaves, and radio waves exhibit similar properties.
The electromagnetic spectrum is the range of frequencies of electromagnetic radiation and their respective wavelengths and photon energies.
In physics, intensity is the power transferred per unit area, where the area is measured on the plane perpendicular to the direction of propagation of the energy. In the SI system, it has units watts per square metre (W/m2). It is used most frequently with waves, in which case the average power transfer over one period of the wave is used. Intensity can be applied to other circumstances where energy is transferred. For example, one could calculate the intensity of the kinetic energy carried by drops of water from a garden sprinkler.
Contents
McPherson specializes in vacuum fabrication, ultraviolet optical systems, spectroscopic technique, and high resolution spectral test and measurement instrumentation. End-user and OEM applications include research, metrology, semiconductors, pharmaceuticals, nanotechnology, aerospace and defense.

Vacuum is space devoid of matter. The word stems from the Latin adjective vacuus for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often discuss ideal test results that would occur in a perfect vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is lower than atmospheric pressure. The Latin term in vacuo is used to describe an object that is surrounded by a vacuum.

Ultraviolet (UV) designates a band of the electromagnetic spectrum with wavelength from 10 nm to 400 nm, shorter than that of visible light but longer than X-rays. UV radiation is present in sunlight, and contributes about 10% of the total light output of the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules.

Spectroscopy is the study of the interaction between matter and electromagnetic radiation. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, by a prism. Later the concept was expanded greatly to include any interaction with radiative energy as a function of its wavelength or frequency, predominantly in the electromagnetic spectrum, though matter waves and acoustic waves can also be considered forms of radiative energy; recently, with tremendous difficulty, even gravitational waves have been associated with a spectral signature in the context of LIGO and laser interferometry. Spectroscopic data are often represented by an emission spectrum, a plot of the response of interest as a function of wavelength or frequency.