Lysine is an α-amino acid that is a precursor to many proteins. It contains an α-amino group, an α-carboxylic acid group, and a side chain lysyl, classifying it as a basic, charged, aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the S configuration.

Penicillium is a genus of ascomycetous fungi that is part of the mycobiome of many species and is of major importance in the natural environment, in food spoilage, and in food and drug production.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Noscapine is a benzylisoquinoline alkaloid, of the phthalideisoquinoline structural subgroup, which has been isolated from numerous species of the family Papaveraceae. It lacks significant hypnotic, euphoric, or analgesic effects affording it with very low addictive potential. This agent is primarily used for its antitussive (cough-suppressing) effects.

Penicillium roqueforti is a common saprotrophic fungus in the genus Penicillium. Widespread in nature, it can be isolated from soil, decaying organic matter, and plants.

The mitomycins are a family of aziridine-containing natural products isolated from Streptomyces caespitosus or Streptomyces lavendulae. They include mitomycin A, mitomycin B, and mitomycin C. When the name mitomycin occurs alone, it usually refers to mitomycin C, its international nonproprietary name. Mitomycin C is used as a medicine for treating various disorders associated with the growth and spread of cells.

The Lysine riboswitch is a metabolite binding RNA element found within certain messenger RNAs that serve as a precision sensor for the amino acid lysine. Allosteric rearrangement of mRNA structure is mediated by ligand binding, and this results in modulation of gene expression. Lysine riboswitch are most abundant in Bacillota and Gammaproteobacteria where they are found upstream of a number of genes involved in lysine biosynthesis, transport and catabolism. The lysine riboswitch has also been identified independently and called the L box.

Betalains are a class of red and yellow tyrosine-derived pigments found in plants of the order Caryophyllales, where they replace anthocyanin pigments. Betalains also occur in some higher order fungi. They are most often noticeable in the petals of flowers, but may color the fruits, leaves, stems, and roots of plants that contain them. They include pigments such as those found in beets.
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:

Ergocryptine is an ergopeptine and one of the ergoline alkaloids. It is isolated from ergot or fermentation broth and it serves as starting material for the production of bromocriptine. Two isomers of ergocryptine exist, α-ergocryptine and β-ergocryptine. The beta differs from the alpha form only in the position of a single methyl group, which is a consequence of the biosynthesis in which the proteinogenic amino acid leucine is replaced by isoleucine. β-Ergocryptine was first identified in 1967 by Albert Hofmann. Ergot from different sources have different ratios of the two isomers.
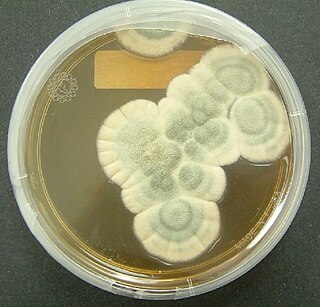
Penicillium chrysogenum is a species of fungus in the genus Penicillium. It is common in temperate and subtropical regions and can be found on salted food products, but it is mostly found in indoor environments, especially in damp or water-damaged buildings. It has been recognised as a species complex that includes P. notatum, P. meleagrinum, and P. cyaneofulvum. Molecular phylogeny has established that Alexander Fleming's first discovered penicillin producing strain is of a distinct species, P. rubens, and not of P. notatum. It has rarely been reported as a cause of human disease. It is the source of several β-lactam antibiotics, most significantly penicillin. Other secondary metabolites of P. chrysogenum include roquefortine C, meleagrin, chrysogine, 6-MSA YWA1/melanin, andrastatin A, fungisporin, secalonic acids, sorbicillin, and PR-toxin.

Penicillium rubens is a species of fungus in the genus Penicillium and was the first species known to produce the antibiotic penicillin. It was first described by Philibert Melchior Joseph Ehi Biourge in 1923. For the discovery of penicillin from this species Alexander Fleming shared the Nobel Prize in Physiology or Medicine in 1945. The original penicillin-producing type has been variously identified as Penicillium rubrum, P. notatum, and P. chrysogenum among others, but genomic comparison and phylogenetic analysis in 2011 resolved that it is P. rubens. It is the best source of penicillins and produces benzylpenicillin (G), phenoxymethylpenicillin (V) and octanoylpenicillin (K). It also produces other important bioactive compounds such as andrastin, chrysogine, fungisporin, roquefortine, and sorbicillins.
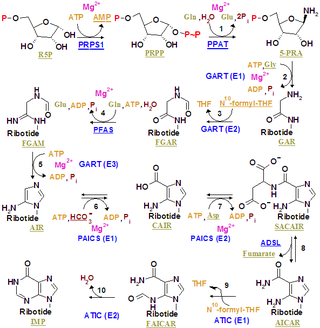
The purinosome is a putative multi-enzyme complex that carries out de novo purine biosynthesis within the cell. It is postulated to include all six of the human enzymes identified as direct participants in this ten-step biosynthetic pathway converting phosphoribosyl pyrophosphate to inosine monophosphate:

15-cis-phytoene desaturases, are enzymes involved in the carotenoid biosynthesis in plants and cyanobacteria. Phytoene desaturases are membrane-bound enzymes localized in plastids and introduce two double bonds into their colorless substrate phytoene by dehydrogenation and isomerize two additional double bonds. This reaction starts a biochemical pathway involving three further enzymes called the poly-cis pathway and leads to the red colored lycopene. The homologous phytoene desaturase found in bacteria and fungi (CrtI) converts phytoene directly to lycopene by an all-trans pathway.
Glucosyl-3-phosphoglycerate synthase is an enzyme with systematic name NDP-glucose:3-phospho-D-glycerate 2-alpha-D-glucosyltransferase. This enzyme catalyses the following chemical reaction

Angomonas deanei is a flagellated trypanosomatid protozoan. As an obligate parasite, it infects the gastrointestinal tract of insects, and is in turn a host to symbiotic bacteria. The bacterial endosymbiont Ca. "Kinetoplastibacterium crithidii" maintains a permanent mutualistic relationship with the protozoan such that it is no longer able to reproduce and survive on its own. The symbiosis, subsequently also discovered in varying degrees in other protists such as Strigomonas culicis, Novymonas esmeraldas, Diplonema japonicumand Diplonema aggregatum are considered as good models for the understanding of the evolution of eukaryotes from prokaryotes, and on the origin of cell organelles.
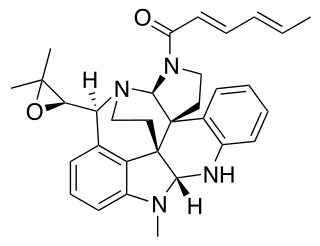
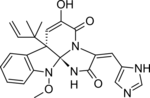
Communesin B is a cytotoxic chemical compound isolated from Penicillium strains found on the marine alga Ulva intestinalis. It exhibits cytotoxicity in vitro against human lung carcinoma, prostate carcinoma, colorectal carcinoma, cervical adenocarcinoma, and breast adenocarcinoma cell lines.
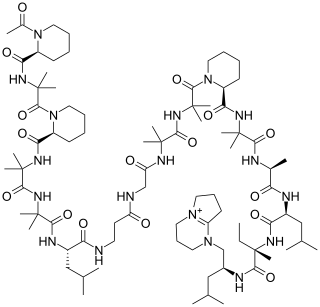
Efrapeptins are peptides produced by fungi in the genus Tolypocladium that have antifungal, insecticidal, and mitochondrial ATPase inhibitory activities. They are produced via a biosynthetic pathway similar to, but simpler than, the ciclosporin pathway with nonribosomal peptide synthase (NRPS) and/or polyketide synthase (PKS) being the key elements.
Butyrolactol A is an organic chemical compound of interest for its potential use as an antifungal antibiotic.
Juvenile hormone acid O-methyltransferase (JHAMT) is a ~33 kDa enzyme that catalyzes the conversion of inactive precursors of Juvenile hormones (JHs) to active JHs in the final stages of JH biosynthesis in the corpora allata of insects. More specifically, the enzyme catalyzes the transfer of a methyl group from S-adenosyl-L-methionine (SAM) to the carboxylate group of JH precursors.