
Coke is a grey, hard, and porous coal-based fuel with a high carbon content. It is made by heating coal or petroleum in the absence of air. Coke is an important industrial product, used mainly in iron ore smelting, but also as a fuel in stoves and forges.

Cast iron is a class of iron–carbon alloys with a carbon content of more than 2% and silicon content around 1–3%. Its usefulness derives from its relatively low melting temperature. The alloying elements determine the form in which its carbon appears: white cast iron has its carbon combined into an iron carbide named cementite, which is very hard, but brittle, as it allows cracks to pass straight through; grey cast iron has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and ductile cast iron has spherical graphite "nodules" which stop the crack from further progressing.

Anthracite, also known as hard coal and black coal, is a hard, compact variety of coal that has a submetallic lustre. It has the highest carbon content, the fewest impurities, and the highest energy density of all types of coal and is the highest ranking of coals.

Steelmaking is the process of producing steel from iron ore and/or scrap. In steelmaking, impurities such as nitrogen, silicon, phosphorus, sulfur, and excess carbon are removed from the sourced iron, and alloying elements such as manganese, nickel, chromium, carbon, and vanadium are added to produce different grades of steel.

A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. Blast refers to the combustion air being supplied above atmospheric pressure.

Induction heating is the process of heating electrically conductive materials, namely metals or semi-conductors, by electromagnetic induction, through heat transfer passing through an inductor that creates an electromagnetic field within the coil to heat up and possibly melt steel, copper, brass, graphite, gold, silver, aluminum, or carbide.

Industrial processes are procedures involving chemical, physical, electrical, or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry.

In materials science, a refractory is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.

An electric arc furnace (EAF) is a furnace that heats material by means of an electric arc.
Ferroalloy refers to various alloys of iron with a high proportion of one or more other elements such as manganese (Mn), aluminium (Al), or silicon (Si). They are used in the production of steels and alloys. The alloys impart distinctive qualities to steel and cast iron or serve important functions during production and are, therefore, closely associated with the iron and steel industry, the leading consumer of ferroalloys. The leading producers of ferroalloys in 2014 were China, South Africa, India, Russia and Kazakhstan, which accounted for 84% of the world production. World production of ferroalloys was estimated as 52.8 million tonnes in 2015.

Puddling is the process of converting pig iron to bar (wrought) iron in a coal fired reverberatory furnace. It was developed in England during the 1780s. The molten pig iron was stirred in a reverberatory furnace, in an oxidizing environment to burn the carbon, resulting in wrought iron. It was one of the most important processes for making the first appreciable volumes of valuable and useful bar iron without the use of charcoal. Eventually, the furnace would be used to make small quantities of specialty steels.

Hot blast refers to the preheating of air blown into a blast furnace or other metallurgical process. As this considerably reduced the fuel consumed, hot blast was one of the most important technologies developed during the Industrial Revolution. Hot blast also allowed higher furnace temperatures, which increased the capacity of furnaces.

A delayed coker is a type of coker whose process consists of heating a residual oil feed to its thermal cracking temperature in a furnace with multiple parallel passes. This cracks the heavy, long chain hydrocarbon molecules of the residual oil into coker gas oil and petroleum coke.

A cupola or cupola furnace is a melting device used in foundries that can be used to melt cast iron, Ni-resist iron and some bronzes. The cupola can be made almost any practical size. The size of a cupola is expressed in diameters and can range from 1.5 to 13 feet. The overall shape is cylindrical and the equipment is arranged vertically, usually supported by four legs. The overall look is similar to a large smokestack.
Pulverized coal injection is a method for improving the performance of a blast furnace.
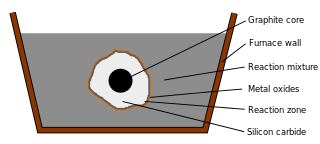
The Acheson process is a method of synthesizing silicon carbide (SiC) and graphite invented by Edward Goodrich Acheson and patented by him in 1896.
In 2022, the U.S. was the third-largest producer of raw steel worldwide, after China and India, and ranked sixth in pig iron production. By November 2024, the industry produced over 74 million net tons annually.

A metallurgical furnace, often simply referred to as a furnace when the context is known, is an industrial furnace used to heat, melt, or otherwise process metals. Furnaces have been a central piece of equipment throughout the history of metallurgy; processing metals with heat is even its own engineering specialty known as pyrometallurgy.
Adrien C. B. Chenot was a French engineer best known for his inventions in metallurgy as well as his research on manufactured gases. He is notably the inventor of one of the first modern methods of direct reduction of iron ore, based on the use of coal reacting with the ore in retorts. He exhibited the first samples of pre-reduced iron ore at the Lisbon Universal Exhibition of 1849, and was awarded the "Grandes Medailles d'Or" at the Paris Universal Exposition of 1855.

A coking factory or a coking plant is where coke and manufactured gas are synthesized from coal using a dry distillation process. The volatile components of the pyrolyzed coal, released by heating to a temperature of between 900°C and 1,400 °C, are generally drawn off and recovered. There are also coking plants where the released components are burned: this is known as a heat recovery process. A layer of ash then forms on the surface of the resulting coke. The degassing of the coal gives the coke a highly sought-after porosity. The gases are broken down by fractional condensation into hydrocarbon tars, sulfuric acid, ammonia, naphthalene, benzol, and coke gas; these products are then purified in further chemical reactors. Germany still has five coking plants in operation to meet the needs of its domestic industry.

















