
In atomic physics, the Rutherford–Bohr model or Bohr model or Bohr diagram, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by revolving electrons —similar to the structure of the Solar System, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.

A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, corresponding to a stable electronic configuration. In organic chemistry covalent bonds are much more common than ionic bonds.
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly, also being applied to polyatomic ions.

The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the center of the nucleus to the boundary of the surrounding shells of electrons. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Three widely used definitions of atomic radius are: Van der Waals radius, ionic radius, and covalent radius.

A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized.
Ionization or ionisation, is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.

In physics and chemistry, ionization energy (American English spelling) or ionisation energy (British English spelling), denoted Ei, is the minimum amount of energy required to remove the most loosely bound electron, the valence electron, of an isolated neutral gaseous atom or molecule. It is quantitatively expressed as
Noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon. From the standpoint of chemistry, the noble gases may be divided into two groups: the relatively reactive krypton, xenon (12.1 eV), and radon (10.7 eV) on one side, and the very unreactive argon (15.8 eV), neon (21.6 eV), and helium (24.6 eV) on the other. Consistent with this classification, Kr, Xe, and Rn form compounds that can be isolated in bulk at or near standard temperature and pressure, whereas He, Ne, Ar have been observed to form true chemical bonds using spectroscopic techniques, but only when frozen into a noble gas matrix at temperatures of 40 K or lower, in supersonic jets of noble gas, or under extremely high pressures with metals.
In chemistry, valence bond (VB) theory is one of two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds when a molecule is formed. In contrast, molecular orbital theory has orbitals that cover the whole molecule.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a Penning mixture to improve the electrical characteristics of the lamps.

The helium hydride ion or hydridohelium(1+) ion is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be one of the first compounds formed in the Universe after the Big Bang.

Positronium hydride, or hydrogen positride is an exotic molecule consisting of a hydrogen atom bound to an exotic atom of positronium. Its formula is PsH. It was predicted to exist in 1951 by A Ore, and subsequently studied theoretically, but was not observed until 1990. R. Pareja, R. Gonzalez from Madrid trapped positronium in hydrogen laden magnesia crystals. The trap was prepared by Yok Chen from the Oak Ridge National Laboratory. In this experiment the positrons were thermalized so that they were not traveling at high speed, and they then reacted with H− ions in the crystal. In 1992 it was created in an experiment done by David M. Schrader and F.M. Jacobsen and others at the Aarhus University in Denmark. The researchers made the positronium hydride molecules by firing intense bursts of positrons into methane, which has the highest density of hydrogen atoms. Upon slowing down, the positrons were captured by ordinary electrons to form positronium atoms which then reacted with hydrogen atoms from the methane.
The dihydrogen cation or hydrogen molecular ion is a cation with formula H+
2. It consists of two hydrogen nuclei (protons) sharing a single electron. It is the simplest molecular ion.
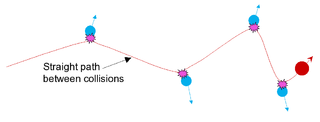
The binary collision approximation (BCA) signifies a method used in ion irradiation physics to enable efficient computer simulation of the penetration depth and defect production by energetic ions in solids. In the method, the ion is approximated to travel through a material by experiencing a sequence of independent binary collisions with sample atoms (nuclei). Between the collisions, the ion is assumed to travel in a straight path, experiencing electronic stopping power, but losing no energy in collisions with nuclei.
An excimer lamp is a source of ultraviolet light produced by spontaneous emission of excimer (exciplex) molecules.
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
Helium is the most unreactive element, so it was commonly believed that helium compounds do not exist at all. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons or join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. The atom is very hard with a Pearson's hardness of 12.3 eV. It has the lowest polarizability of any kind of atom. However very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces. So at extremely low temperatures helium may form van der Waals molecules.
Neon compounds are chemical compounds containing the element neon (Ne) with other molecules or elements from the periodic table. Compounds of the noble gas neon were believed not to exist, but there are now known to be molecular ions containing neon, as well as temporary excited neon-containing molecules called excimers. Several neutral neon molecules have also been predicted to be stable, but are yet to be discovered in nature. Neon has been shown to crystallise with other substances and form clathrates or Van der Waals solids.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by CSIRO.
The magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion. The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.
















