
Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

In organic chemistry, isothiocyanate is the functional group −N=C=S, formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosinolates. These natural isothiocyanates, such as allyl isothiocyanate, are also known as mustard oils. An artificial isothiocyanate, phenyl isothiocyanate, is used for amino acid sequencing in the Edman degradation.

Gear oil is a lubricant made specifically for transmissions, transfer cases, and differentials in automobiles, trucks, and other machinery. It has high viscosity and usually contains organosulfur compounds. Some modern automatic transaxles do not use a heavy oil at all but lubricate with the lower viscosity hydraulic fluid, which is available at pressure within the automatic transmission. Gear oils account for about 20% of the lubricant market.
In organic chemistry, the Menshutkin reaction converts a tertiary amine into a quaternary ammonium salt by reaction with an alkyl halide. Similar reactions occur when tertiary phosphines are treated with alkyl halides.
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme:
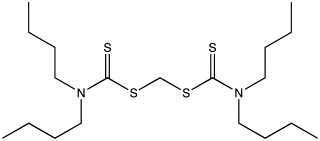
Extreme pressure additives, or EP additives, are additives for lubricants with a role to decrease wear of the parts of the gears exposed to very high pressures. They are also added to cutting fluids for machining of metals.

Methyl isothiocyanate is the organosulfur compound with the formula CH3N=C=S. This low melting colorless solid is a powerful lachrymator. As a precursor to a variety of valuable bioactive compounds, it is the most important organic isothiocyanate in industry.

Sodium diethyldithiocarbamate is the organosulfur compound with the formula NaS2CN(C2H5)2. It is a pale yellow, water soluble salt.

In organic chemistry, a dithiocarbamate is a functional group with the general formula R2N−C(=S)−S−R and structure >N−C(=S)−S−. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms.

Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and R = Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as in the manufacture of pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents.
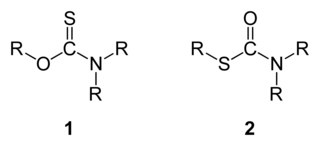
In organic chemistry, thiocarbamates (thiourethanes) are a family of organosulfur compounds. As the prefix thio- suggests, they are sulfur analogues of carbamates. There are two isomeric forms of thiocarbamates: O-thiocarbamates, ROC(=S)NR2 (esters), and S-thiocarbamates, RSC(=O)NR2 (thioesters).
Trifluorotoluene is an organic compound with the formula of C6H5CF3. This colorless fluorocarbon is used as a specialty solvent in organic synthesis and an intermediate in the production of pesticides and pharmaceuticals.

Metam sodium is an organosulfur compound with the formula CH3NHCS2Na. The compound is a sodium salt of a dithiocarbamate. The compound exists as a colorless dihydrate, but most commonly it is encountered as an aqueous solution. It is used as a soil fumigant, pesticide, herbicide, and fungicide. It is one of the most widely used pesticides in the United States, with approximately 60 million pounds used in 2001.
Zineb is the chemical compound with the formula {Zn[S2CN(H)CH2CH2N(H)CS2]}n. Structurally, it is classified as a coordination polymer and a dithiocarbamate complex. This pale yellow solid is used as fungicide.
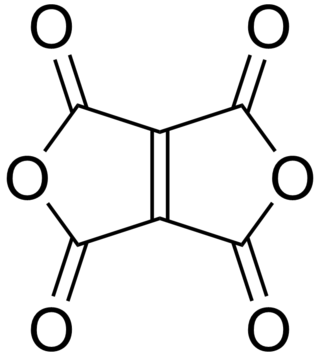
Ethylenetetracarboxylic dianhydride is a chemical compound with formula C
6O
6, that can be seen as the twofold anhydride of ethylenetetracarboxylic acid C
6H
4O
8. It has a bicyclic molecular structure consisting of two maleic anhydride rings fused by their respective alkene units. It is a pale yellow oily liquid, soluble in dichloromethane and chloroform.

Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide.
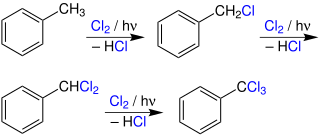
Photochlorination is a chlorination reaction that is initiated by light. Usually a C-H bond is converted to a C-Cl bond. Photochlorination is carried out on an industrial scale. The process is exothermic and proceeds as a chain reaction initiated by the homolytic cleavage of molecular chlorine into chlorine radicals by ultraviolet radiation. Many chlorinated solvents are produced in this way.

Iron tris(diethyldithiocarbamate) is the coordination complex of iron with diethyldithiocarbamate with the formula Fe(S2CNEt2)3 (Et = ethyl). It is a black solid that is soluble in organic solvents.

Cobalt tris(diethyldithiocarbamate) is the coordination complex of cobalt with diethyldithiocarbamate with the formula Co(S2CNEt2)3 (Et = ethyl). It is a diamagnetic green solid that is soluble in organic solvents.

Transition metal dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known. Several homoleptic derivatives have the formula M(R2dtc)n where n = 2 and 3.















