
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is their cell envelope, which consists of a thin peptidoglycan cell wall sandwiched between an inner (cytoplasmic) membrane and an outer membrane. These bacteria are found in all environments that support life on Earth.

Bdellovibrio is a genus of Gram-negative, obligate aerobic bacteria. One of the more notable characteristics of this genus is that members can prey upon other Gram-negative bacteria and feed on the biopolymers, e.g. proteins and nucleic acids, of their hosts. They have two lifestyles: a host-dependent, highly mobile phase, the "attack phase", in which they form "bdelloplasts" in their host bacteria; and a slow-growing, irregularly shaped, host-independent form.
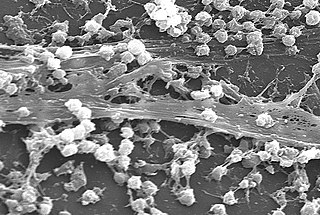
A biofilm is an syntrophic community of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric combination of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes".
In biology, quorum sensing or quorum signaling (QS) is the ability to detect and respond to cell population density by gene regulation. Quorum sensing is a type of cellular signaling, and more specifically can be considered a type of paracrine signaling. However, it also contains traits of both autocrine signaling: a cell produces both the autoinducer molecule and the receptor for the autoinducer. As one example, QS enables bacteria to restrict the expression of specific genes to the high cell densities at which the resulting phenotypes will be most beneficial, especially for phenotypes that would be ineffective at low cell densities and therefore too energetically costly to express. Many species of bacteria use quorum sensing to coordinate gene expression according to the density of their local population. In a similar fashion, some social insects use quorum sensing to determine where to nest. Quorum sensing in pathogenic bacteria activates host immune signaling and prolongs host survival, by limiting the bacterial intake of nutrients, such as tryptophan, which further is converted to serotonin. As such, quorum sensing allows a commensal interaction between host and pathogenic bacteria. Quorum sensing may also be useful for cancer cell communications.

Pseudomonas is a genus of Gram-negative bacteria belonging to the family Pseudomonadaceae in the class Gammaproteobacteria. The 313 members of the genus demonstrate a great deal of metabolic diversity and consequently are able to colonize a wide range of niches. Their ease of culture in vitro and availability of an increasing number of Pseudomonas strain genome sequences has made the genus an excellent focus for scientific research; the best studied species include P. aeruginosa in its role as an opportunistic human pathogen, the plant pathogen P. syringae, the soil bacterium P. putida, and the plant growth-promoting P. fluorescens, P. lini, P. migulae, and P. graminis.

Phage therapy, viral phage therapy, or phagotherapy is the therapeutic use of bacteriophages for the treatment of pathogenic bacterial infections. This therapeutic approach emerged at the beginning of the 20th century but was progressively replaced by the use of antibiotics in most parts of the world after the Second World War. Bacteriophages, known as phages, are a form of virus that attach to bacterial cells and inject their genome into the cell. The bacteria's production of the viral genome interferes with its ability to function, halting the bacterial infection. The bacterial cell causing the infection is unable to reproduce and instead produces additional phages. Phages are very selective in the strains of bacteria they are effective against.

Aztreonam, sold under the brand name Azactam among others, is an antibiotic used primarily to treat infections caused by gram-negative bacteria such as Pseudomonas aeruginosa. This may include bone infections, endometritis, intra abdominal infections, pneumonia, urinary tract infections, and sepsis. It is given by intravenous or intramuscular injection or by inhalation.

Colistin, also known as polymyxin E, is an antibiotic medication used as a last-resort treatment for multidrug-resistant Gram-negative infections including pneumonia. These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter. It comes in two forms: colistimethate sodium can be injected into a vein, injected into a muscle, or inhaled, and colistin sulfate is mainly applied to the skin or taken by mouth. Colistimethate sodium is a prodrug; it is produced by the reaction of colistin with formaldehyde and sodium bisulfite, which leads to the addition of a sulfomethyl group to the primary amines of colistin. Colistimethate sodium is less toxic than colistin when administered parenterally. In aqueous solutions it undergoes hydrolysis to form a complex mixture of partially sulfomethylated derivatives, as well as colistin. Resistance to colistin began to appear as of 2015.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. P. aeruginosa is able to selectively inhibit various antibiotics from penetrating its outer membrane - and has high resistance to several antibiotics, according to the World Health Organization P. aeruginosa poses one of the greatest threats to humans in terms of antibiotic resistance.
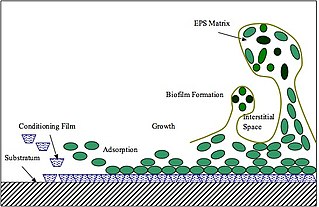
Extracellular polymeric substances (EPSs) are natural polymers of high molecular weight secreted by microorganisms into their environment. EPSs establish the functional and structural integrity of biofilms, and are considered the fundamental component that determines the physicochemical properties of a biofilm. EPS in the matrix of biofilms provides compositional support and protection of microbial communities from the harsh environments. Components of EPS can be of different classes of polysaccharides, lipids, nucleic acids, proteins, lipopolysaccharides, and minerals.
Vampirococcus is an informally described genus of ovoid Gram-negative bacteria, but the exact phylogeny remains to be determined. This predatory prokaryote was first described in 1983 by Esteve et al. as small, anaerobic microbe about 0.6 μm wide before being given the name of Vampirococcus in 1986 by Guerrero et al. This prokaryote is a freshwater obligate predator that preys specifically on various species of the photosynthetic purple sulfur bacterium, Chromatium. As an epibiont, Vampirococcus attaches to the cell surface of their prey and "sucks" out the cytoplasm using a specialized cytoplasmic bridge. They are commonly mentioned as an example of epibionts when discussing strategies employed by bacterial predators. This microbe still has yet to be classified based on genomic sequencing or 16S rRNA because it cannot be sustained long enough outside its natural environment to isolate a pure culture.
Delftia tsuruhatensis is a Gram-negative, rod-shaped, catalase- and oxidase-positive, motile bacterium from the Comamonadaceae family. It was first isolated from a wastewater treatment plant in Japan in 2003. D. tsuruhatensis is an opportunistic and emergent pathogen. All documented human infections are healthcare-associated.
Bacteriovorax is a genus containing a single species of bacterium in the family Bacteriovoracaceae, Bacteriovorax stolpii. It is a predator that feeds on larger Gram-negative bacteria. These prey bacteria tend to live in enteric environments and have similar lipopolysaccharide structures. Bacteriovorax stolpii recognizes its prey by outer membrane protein receptors, which explains why Gram-positive bacteria that lack outer membranes do not serve as prey. They prey on bacteria by invading the interperiplasmic space where they feed, grow, and reproduce. Bacteriovorax stolpii used to be classified in the genus Bdellovibrio because of similar morphologies and lifestyle characteristics, however they were recognized as a new genus through phylogenetic analysis.

Vampirovibrio chlorellavorus is a 0.6 µm pleomorphic cocci with a gram negative cell wall, and is one of the few known predatory bacteria. Unlike many bacteria, V. chlorellavorus is an obligate parasite, attaching to the cell wall of green algae of the genus Chlorella. The name Vampirovibrio originates from the Serbian vampir. meaning vampire and vibrio referring to the bacterial genus of curved rod bacterium. Chlorellavorus is named for the algal host of the bacterium (Chlorella) and the Latin voro meaning "to devour" (Chlorella-devouring).

Caenorhabditis elegans- microbe interactions are defined as any interaction that encompasses the association with microbes that temporarily or permanently live in or on the nematode C. elegans. The microbes can engage in a commensal, mutualistic or pathogenic interaction with the host. These include bacterial, viral, unicellular eukaryotic, and fungal interactions. In nature C. elegans harbours a diverse set of microbes. In contrast, C. elegans strains that are cultivated in laboratories for research purposes have lost the natural associated microbial communities and are commonly maintained on a single bacterial strain, Escherichia coli OP50. However, E. coli OP50 does not allow for reverse genetic screens because RNAi libraries have only been generated in strain HT115. This limits the ability to study bacterial effects on host phenotypes. The host microbe interactions of C. elegans are closely studied because of their orthologs in humans. Therefore, the better we understand the host interactions of C. elegans the better we can understand the host interactions within the human body.

Twitching motility is a form of crawling bacterial motility used to move over surfaces. Twitching is mediated by the activity of hair-like filaments called type IV pili which extend from the cell's exterior, bind to surrounding solid substrates, and retract, pulling the cell forwards in a manner similar to the action of a grappling hook. The name twitching motility is derived from the characteristic jerky and irregular motions of individual cells when viewed under the microscope. It has been observed in many bacterial species, but is most well studied in Pseudomonas aeruginosa, Neisseria gonorrhoeae and Myxococcus xanthus. Active movement mediated by the twitching system has been shown to be an important component of the pathogenic mechanisms of several species.
Renee Elizabeth Sockett is a professor and microbiologist in the School of Life Sciences at the University of Nottingham. She is a world-leading expert on Bdellovibrio bacteriovorus, a species of predatory bacteria.
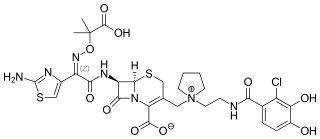
Cefiderocol, sold under the brand name Fetroja among others, is an antibiotic used to treat complicated urinary tract infections when no other options are available. It is indicated for the treatment of multi-drug-resistant Gram-negative bacteria including Pseudomonas aeruginosa. It is given by injection into a vein.

Alain Ange-Marie Filloux is a French/British microbiologist who is a Professor of Molecular Microbiology at Imperial College London. His research looks at the chronic infection of Pseudomonas aeruginosa, a Gram-negative bacterium that causes nosocomial infections in people who are immunocompromised and a deadly threat for cystic fibrosis patients.
The molecule 2-heptyl-3-hydroxy-4-quinolone, also named the Pseudomonas quinolone signal (PQS), has been discovered as an intracellular link between the two major quorum sensing systems of P. aeruginosa; the las and rhl systems. These systems together control expression of virulence factors and play a major role in the formation of biofilms in Pseudomonas aeruginosa. P. aeruginosa is a gram-negative bacteria and opportunistic human pathogen that can cause serious and sometimes fatal infections in humans. Similar to other bacterial species, P. aeruginosa uses quorum sensing (QS) systems to communicate between cells in a population. This allows coordination of gene expression in a population based on changing cell densities, abundance of nutrients, and other environmental factors.














