Related Research Articles

Quantum dots (QDs) – also called semiconductor nanocrystals, are semiconductor particles a few nanometres in size, having optical and electronic properties that differ from those of larger particles as a result of quantum mechanics. They are a central topic in nanotechnology and materials science. When the quantum dots are illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission (photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band, or the transition between discrete energy states when the band structure is no longer well-defined in QDs.
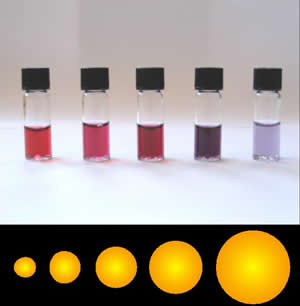
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is coloured usually either wine red or blue-purple . Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.

A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead.
A nanocrystal is a material particle having at least one dimension smaller than 100 nanometres, based on quantum dots and composed of atoms in either a single- or poly-crystalline arrangement.
Hydrogen-terminated silicon surface is a chemically passivated silicon substrate where the surface Si atoms are bonded to hydrogen. The hydrogen-terminated surfaces are hydrophobic, luminescent, and amenable to chemical modification. Hydrogen-terminated silicon is an intermediate in the growth of bulk silicon from silane:
Porous silicon is a form of the chemical element silicon that has introduced nanopores in its microstructure, rendering a large surface to volume ratio in the order of 500 m2/cm3.
Gold Nanocages are hollow, porous gold nanoparticles ranging in size from 10 to over 150 nm. They are created by reacting silver nanoparticles with chloroauric acid (HAuCl4) in boiling water. Whereas gold nanoparticles absorb light in the visible spectrum of light (at about 550 nm), gold nanocages absorb light in the near-infrared, where biological tissues absorb the least light. Because they are also biocompatible, gold nanocages are promising as a contrast agent for optical coherence tomography. Gold nanocages also absorb light and heat up (Photothermal effect), killing surrounding cancer cells. Nanocages have been functionalized with cancer-specific antibodies.

Electrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes.

Thalappil Pradeep is an institute professor and professor of chemistry in the Department of Chemistry at the Indian Institute of Technology Madras. He is also the Deepak Parekh Chair Professor. In 2020 he received the Padma Shri award for his distinguished work in the field of Science and Technology. He has received the Nikkei Asia Prize (2020), The World Academy of Sciences (TWAS) prize (2018), and the Shanti Swarup Bhatnagar Prize for Science and Technology in 2008 by Council of Scientific and Industrial Research.
Carbide-derived carbon (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as MAX phases (e.g., Ti2AlC, Ti3SiC2). CDCs have also been derived from polymer-derived ceramics such as Si-O-C or Ti-C, and carbonitrides, such as Si-N-C. CDCs can occur in various structures, ranging from amorphous to crystalline carbon, from sp2- to sp3-bonded, and from highly porous to fully dense. Among others, the following carbon structures have been derived from carbide precursors: micro- and mesoporous carbon, amorphous carbon, carbon nanotubes, onion-like carbon, nanocrystalline diamond, graphene, and graphite. Among carbon materials, microporous CDCs exhibit some of the highest reported specific surface areas (up to more than 3000 m2/g). By varying the type of the precursor and the CDC synthesis conditions, microporous and mesoporous structures with controllable average pore size and pore size distributions can be produced. Depending on the precursor and the synthesis conditions, the average pore size control can be applied at sub-Angstrom accuracy. This ability to precisely tune the size and shapes of pores makes CDCs attractive for selective sorption and storage of liquids and gases (e.g., hydrogen, methane, CO2) and the high electric conductivity and electrochemical stability allows these structures to be effectively implemented in electrical energy storage and capacitive water desalinization.

Professor Warren Chan is a full professor at the Institute of Biomedical Engineering and Terrence Donnelly Centre for Cellular and Biomolecular Research at the University of Toronto. He received his B.S. and PhD degree, and post-doctoral training from the University of Illinois, Indiana University, and University of California, San Diego.

Carbon quantum dots also commonly called carbon dots are carbon nanoparticles which are less than 10 nm in size and have some form of surface passivation.
Niveen M. Khashab is a Lebanese chemist and an associate Professor of chemical Sciences and engineering at King Abdullah University of Science and Technology in Saudi Arabia since 2009. She is a laureate of the 2017 L'Oréal-UNESCO Awards for Women in Science "for her contributions to innovative smart hybrid materials aimed at drug delivery and for developing new techniques to monitor intracellular antioxidant activity." She is also a fellow of the Royal Chemical Society, and a member of the American Chemical Society.
Upconverting nanoparticles (UCNPs) are nanoscale particles that exhibit photon upconversion. In photon upconversion, two or more incident photons of relatively low energy are absorbed and converted into one emitted photon with higher energy. Generally, absorption occurs in the infrared, while emission occurs in the visible or ultraviolet regions of the electromagnetic spectrum. UCNPs are usually composed of rare-earth based lanthanide- or actinide-doped transition metals and are of particular interest for their applications in in vivo bio-imaging, bio-sensing, and nanomedicine because of their highly efficient cellular uptake and high optical penetrating power with little background noise in the deep tissue level. They also have potential applications in photovoltaics and security, such as infrared detection of hazardous materials.
Quantum dots (QDs) are semiconductor nanoparticles with a size less than 10 nm. They exhibited size-dependent properties especially in the optical absorption and the photoluminescence (PL). Typically, the fluorescence emission peak of the QDs can be tuned by changing their diameters. So far, QDs were consisted of different group elements such as CdTe, CdSe, CdS in the II-VI category, InP or InAs in the III-V category, CuInS2 or AgInS2 in the I–III–VI2 category, and PbSe/PbS in the IV-VI category. These QDs are promising candidates as fluorescent labels in various biological applications such as bioimaging, biosensing and drug delivery.

Irshad Hussain is a Pakistani Scientist in the field of chemistry and among the few pioneers to initiate nanomaterials research in Pakistan.
Kimberly A. Prather is an American atmospheric chemist. She is a distinguished chair in atmospheric chemistry and a distinguished professor at the Scripps Institution of Oceanography and department of chemistry and biochemistry at UC San Diego. Her work focuses on how humans are influencing the atmosphere and climate. In 2019, she was elected a member of the National Academy of Engineering for technologies that transformed understanding of aerosols and their impacts on air quality, climate, and human health. In 2020, she was elected as a member of the National Academy of Sciences. She is also an elected Fellow of the American Philosophical Society, American Geophysical Union, the American Association for the Advancement of Science, American Philosophical Society, and the American Academy of Arts and Sciences.
Susan M. Kauzlarich is an American chemist and is presently a distinguished professor of chemistry at the University of California, Davis. At UC Davis, Kauzlarich leads a research group focused on the synthesis and characterization of Zintl phases and nanoclusters with applications in the fields of thermoelectric materials, magnetic resonance imaging, energy storage, opto-electronics, and drug delivery. Kauzlarich has published over 250 peer-reviewed publications and has been awarded several patents. In 2009, Kauzlarich received the annual Presidential Award for Excellence in Science, Mathematics and Engineering Mentoring, which is administered by the National Science Foundation to acknowledge faculty members who raise the membership of minorities, women and disabled students in the science and engineering fields. In January 2022 she became Deputy Editor for the scientific journal, Science Advances.
Silicon quantum dots are metal-free biologically compatible quantum dots with photoluminescence emission maxima that are tunable through the visible to near-infrared spectral regions. These quantum dots have unique properties arising from their indirect band gap, including long-lived luminescent excited-states and large Stokes shifts. A variety of disproportionation, pyrolysis, and solution protocols have been used to prepare silicon quantum dots, however it is important to note that some solution-based protocols for preparing luminescent silicon quantum dots actually yield carbon quantum dots instead of the reported silicon. The unique properties of silicon quantum dots lend themselves to an array of potential applications: biological imaging, luminescent solar concentrators, light emitting diodes, sensors, and lithium-ion battery anodes.

Liangfang Zhang is a Chinese-American nanoengineer. He is the Chancellor Professor of Nanoengineering and Bioengineering and Director of Chemical Engineering at the University of California, San Diego. Zhang is a Fellow of the American Institute for Medical and Biological Engineering, American Association for the Advancement of Science, and the National Academy of Inventors.
References
- ↑ "Michael J. Sailor Biographical". UCSD Sailor Research Group. University of California, San Diego.
- ↑ Sailor, Michael J. (2012). Porous silicon in practice preparation, characterization and applications. Weinheim: Wiley-VCH. p. 249. ISBN 9783527313785.
- ↑ Lin, V.S.Y.; Motesharei, K.; Sailor, M. J.; Ghadiri, M. R. (31 October 1997). "A Porous Silicon-Based Optical Interferometric Biosensor". Science. 278 (5339): 840–843. Bibcode:1997Sci...278..840L. doi:10.1126/science.278.5339.840. PMID 9346478.
- ↑ Heinrich, J.L.; Curtis, C.L.; Credo, G.M.; Kavanagh, K.L.; Sailor, M.J. (3 January 1992). "Luminescent colloidal Si suspensions from porous Si". Science. 255 (5040): 66–68. doi:10.1126/science.255.5040.66. PMID 17739915. S2CID 19694068.
- ↑ Park, J.H.; Gu, L.; Rouslahti, E.; Bhatia, S.N.; Sailor, M.J. (22 February 2009). "Biodegradable luminescent porous silicon nanoparticles for in vivo applications". Nature Materials. 8 (4): 331–336. Bibcode:2009NatMa...8..331P. doi:10.1038/nmat2398. PMC 3058936 . PMID 19234444.
- ↑ Gu, L.; Hall, D.J.; Qin, Z.; Anglin, E.; Joo, J.; Mooney, D.J.; Howell, S.B.; Sailor, M.J. (12 August 2013). "In vivo time-gated fluorescence imaging with biodegradable luminescent porous silicon nanoparticles". Nature Communications. 4: 2326. Bibcode:2013NatCo...4.2326G. doi:10.1038/ncomms3326. PMC 4154512 . PMID 23933660.
- ↑ Sailor, M.J.; Link, J.R. (10 Feb 2005). "Smart Dust: nanostructured devices in a grain of sand". Chemical Communications (11): 1375–1383. doi:10.1039/b417554a. PMID 15756310.
- ↑ Dovree, J.R.; Derfus, A.M.; Bhatia, S.N.; Sailor, M.J. (7 November 2004). "Manipulation of liquid droplets using amphiphilic, magnetic 1-D photonic crystal chaperones". Nature Materials. 3 (12): 896–899. doi:10.1038/nmat1253. PMID 15531887. S2CID 8177935.
- ↑ Link, J.R.; Sailor, M.J. (June 19, 2003). "Smart Dust: Self-assembling, self-orienting photonic crystals of porous Si". Proceedings of the National Academy of Sciences. 100 (19): 10607–10610. Bibcode:2003PNAS..10010607L. doi: 10.1073/pnas.1233824100 . PMC 196851 . PMID 12947036.
- ↑ Schmedake, T.A.; Cunin, F.; Link, J.R.; Sailor, M.J. (16 September 2002). "Standoff detection of chemicals using porous silicon 'Smart Dust' particles". Advanced Materials. 14 (18): 1270–1272. doi:10.1002/1521-4095(20020916)14:18<1270::AID-ADMA1270>3.0.CO;2-R.
- ↑ "Harvey Mudd College Alumni Association - Award Recipients". Harvey Mudd College.
- ↑ "Office of Post Doctoral & Visiting Scholar Affairs" . Retrieved 26 October 2014.
- ↑ Dabney, Michael. "Georgia Sadler And Michael Sailor Honored As Outstanding Faculty Mentors To Students". UCSD News. Retrieved 26 October 2014.
- 1 2 3 4 5 "Michael Sailor Ph.D: Executive Profile & Biography - Businessweek". Bloomberg Businessweek.
- ↑ McDonald, Kim. "UCSD Student Wins $50,000 Collegiate Inventors Grand Prize". UCSD News. Retrieved 26 October 2014.
- ↑ Paiva, Rini. "2003 winners of Collegiate Inventors Competition announced in NYC". EurekAlert.
- ↑ "Past Fellows". Alfred Sloan B. Foundation.
- ↑ "Michael J. Sailor". Arnold and Mabel Beckman Foundation. Retrieved 9 March 2017.