
A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

A dendritic cell (DC) is an antigen-presenting cell of the mammalian immune system. A DC's main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and adaptive immune systems.

A monoclonal antibody is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.

In molecular biology, CD4 is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as helper T cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.

Cluster of differentiation 40, CD40 is a type I transmembrane protein found on antigen-presenting cells and is required for their activation. The binding of CD154 (CD40L) on TH cells to CD40 activates antigen presenting cells and induces a variety of downstream effects.

Antonio Lanzavecchia is an Italian and Swiss immunologist. As a fellow of Collegio Borromeo he obtained a degree with honors in Medicine in 1976 from the University of Pavia where he specialized in Pediatrics and Infectious Diseases. He is Head Human Immunology Program, Istituto Nazionale di Genetica Molecolare-INGM, Milan and SVP Senior research Fellow, Humabs/Vir Biotechnology, Bellinzona and San Francisco (USA). Since 2017, he is also Professor at the Faculty of Biomedical Sciences of the Università della Svizzera italiana (USI).

Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication. The suboptimal antibodies can result from natural infection or from vaccination. ADE may cause enhanced respiratory disease, but is not limited to respiratory disease. It has been observed in HIV, RSV, and Dengue virus and is monitored for in vaccine development.
CD4 immunoadhesin is a recombinant fusion protein consisting of a combination of CD4 and the fragment crystallizable region, similarly known as immunoglobulin. It belongs to the antibody (Ig) gene family. CD4 is a surface receptor for human immunodeficiency virus (HIV). The CD4 immunoadhesin molecular fusion allow the protein to possess key functions from each independent subunit. The CD4 specific properties include the gp120-binding and HIV-blocking capabilities. Properties specific to immunoglobulin are the long plasma half-life and Fc receptor binding. The properties of the protein means that it has potential to be used in AIDS therapy as of 2017. Specifically, CD4 immunoadhesin plays a role in antibody-dependent cell-mediated cytotoxicity (ADCC) towards HIV-infected cells. While natural anti-gp120 antibodies exhibit a response towards uninfected CD4-expressing cells that have a soluble gp120 bound to the CD4 on the cell surface, CD4 immunoadhesin, however, will not exhibit a response. One of the most relevant of these possibilities is its ability to cross the placenta.

Ralph Marvin Steinman was a Canadian physician and medical researcher at Rockefeller University, who in 1973 discovered and named dendritic cells while working as a postdoctoral fellow in the laboratory of Zanvil A. Cohn, also at Rockefeller University. Steinman was one of the recipients of the 2011 Nobel Prize in Physiology or Medicine.
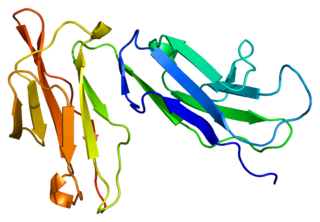
Fc fragment of IgG receptor IIb is a low affinity inhibitory receptor for the Fc region of immunoglobulin gamma (IgG). FCGR2B participates in the phagocytosis of immune complexes and in the regulation of antibody production by B lymphocytes.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy.
Barton Ford Haynes is an American physician and immunologist internationally recognized for work in T-cell immunology, retrovirology, and HIV vaccine development. Haynes is a Frederic M. Hanes Professor of Medicine and Immunology at Duke University Medical Center. He is the director of the Duke Human Vaccine Institute and the Duke Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID), which was funded by the National Institute of Allergy and Infectious Diseases (NIAID) in 2012. In addition, Haynes directs the B-cell Lineage Envelope Design Study, the Centralized Envelope Phase I Study, and the Role of IgA in HIV-1 Protection Study as part of the Collaboration for AIDS Vaccine Discovery (CAVD), which was funded by the Bill and Melinda Gates Foundation in 2006.
J. (Joseph) Donald Capra was an American immunologist, physician-scientist, and was the 4th full-time president (1997–2007) and later, president emeritus, of the Oklahoma Medical Research Foundation (OMRF) in Oklahoma City, OK. While president, he helped to raise over $100 million and spearheaded major research growth in grants funded and faculty recruited to the institution.

Susan Zolla-Pazner is an American research scientist who is a Professor of Medicine in the Division of Infectious Diseases and the Department of Microbiology at Mount Sinai School of Medicine and a guest investigator in the Laboratory of Molecular Immunology at The Rockefeller University, both in New York City. Zolla-Pazner's work has focused on how the immune system responds to the human immunodeficiency virus (HIV) and, in particular, how antibodies against the viral envelope develop in the course of infection.
Intrastructural help (ISH) is where T and B cells cooperate to help or suppress an immune response gene. ISH has proven effective for the treatment of influenza, rabies related lyssavirus, hepatitis B, and the HIV virus. This process was used in 1979 to observe that T cells specific to the influenza virus could promote the stimulation of hemagglutinin specific B cells and elicit an effective humoral immune response. It was later applied to the lyssavirus and was shown to protect raccoons from lethal challenge. The ISH principle is especially beneficial because relatively invariable structural antigens can be used for the priming of T-cells to induce humoral immune response against variable surface antigens. Thus, the approach has also transferred well for the treatment of hepatitis B and HIV.
Marina Fernandes De Barros Caskey is a Brazilian Physician-scientist, immunologist and professor at Rockefeller University.

Nina Papavasiliou is an immunologist and Helmholtz Professor in the Division of Immune Diversity at the German Cancer Research Center in Heidelberg, Germany. She is also an adjunct professor at the Rockefeller University, where she was previously associate professor and head of the Laboratory of Lymphocyte Biology. She is best known for her work in the fields of DNA and RNA editing.
Oligoclonal antibodies are an emerging immunological treatment relying on the combinatory use of several monoclonal antibodies (mAb) in one single drug. The composition can be made of mAb targeting different epitopes of a same protein (homo-combination) or mAb targeting different proteins (hetero-combination). It mimicks the natural polyclonal humoral immunological response to get better efficiency of the treatment. This strategy is most efficient in infections and in cancer treatment as it allow to overcome acquired resistance by pathogens and the plasticity of cancers.

Moriya Tsuji is an American immunologist and vaccinologist. As of 2024, he serves as a Professor of Medicine in the Division of Infectious Diseases, Department of Medicine, and at the Aaron Diamond AIDS Research Center.













