
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells which generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.
A regenerative fuel cell or reverse fuel cell (RFC) is a fuel cell run in reverse mode, which consumes electricity and chemical B to produce chemical A. By definition, the process of any fuel cell could be reversed. However, a given device is usually optimized for operating in one mode and may not be built in such a way that it can be operated backwards. Standard fuel cells operated backwards generally do not make very efficient systems unless they are purpose-built to do so as with high-pressure electrolysers, regenerative fuel cells, solid-oxide electrolyser cells and unitized regenerative fuel cells.

Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically-determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency. In an electrolytic cell the existence of overpotential implies that the cell requires more energy than thermodynamically expected to drive a reaction. In a galvanic cell the existence of overpotential means less energy is recovered than thermodynamics predicts. In each case the extra/missing energy is lost as heat. The quantity of overpotential is specific to each cell design and varies across cells and operational conditions, even for the same reaction. Overpotential is experimentally determined by measuring the potential at which a given current density is achieved.
Hydrogen production is the family of industrial methods for generating hydrogen gas. There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis of water; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. As of 2020, the majority of hydrogen (~95%) is produced by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification. Other methods of hydrogen production include biomass gasification and methane pyrolysis. Methane pyrolysis and water electrolysis can use any source of electricity including renewable energy.
Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system also known as micro fuel cell that generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds on the anode to oxidized compounds such as oxygen on the cathode through an external electrical circuit. MFCs produce electricity by using the electrons derived from biochemical reactions catalyzed by bacteria. MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. In the 21st century MFCs have started to find commercial use in wastewater treatment.
Electrohydrogenesis or biocatalyzed electrolysis is the name given to a process for generating hydrogen gas from organic matter being decomposed by bacteria. This process uses a modified fuel cell to contain the organic matter and water. A small amount, 0.2–0.8 V of electricity is used, the original article reports an overall energy efficiency of 288% can be achieved. This work was reported by Cheng and Logan.
In electrochemistry, Faraday efficiency describes the efficiency with which charge (electrons) is transferred in a system facilitating an electrochemical reaction. The word "Faraday" in this term has two interrelated aspects: first, the historic unit for charge is the faraday (F), but has since been replaced by the coulomb (C); and secondly, the related Faraday's constant correlates charge with moles of matter and electrons. This phenomenon was originally understood through Michael Faraday's work and expressed in his laws of electrolysis.
An enzymatic biofuel cell is a specific type of fuel cell that uses enzymes as a catalyst to oxidize its fuel, rather than precious metals. Enzymatic biofuel cells, while currently confined to research facilities, are widely prized for the promise they hold in terms of their relatively inexpensive components and fuels, as well as a potential power source for bionic implants.

A solid oxide electrolyzer cell (SOEC) is a solid oxide fuel cell that runs in regenerative mode to achieve the electrolysis of water by using a solid oxide, or ceramic, electrolyte to produce hydrogen gas and oxygen. The production of pure hydrogen is compelling because it is a clean fuel that can be stored, making it a potential alternative to batteries, methane, and other energy sources. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to thermochemical and photocatalytic methods.
Electromethanogenesis is a form of electrofuel production where methane is produced by direct biological conversion of electrical current and carbon dioxide.
Microbial electrosynthesis (MES) is a form of microbial electrocatalysis in which electrons are supplied to living microorganisms via a cathode in an electrochemical cell by applying an electric current. The electrons are then used by the microorganisms to reduce carbon dioxide to yield industrially relevant products. The electric current would ideally be produced by a renewable source of power. This process is the opposite to that employed in a microbial fuel cell, in which microorganisms transfer electrons from the oxidation of compounds to an anode to generate an electric current.
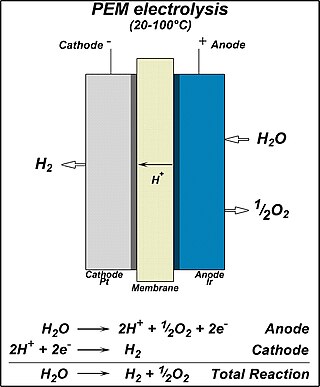
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.

Microbial electrolysis carbon capture (MECC) is a carbon capture technique using microbial electrolysis cells during wastewater treatment. MECC results in net negative carbon emission wastewater treatment by removal of carbon dioxide (CO2) during the treatment process in the form of calcite (CaCO3), and production of profitable H2 gas.

Pulse electrolysis is an alternate electrolysis method that utilises a pulsed direct current to initiate non-spontaneous chemical reactions. Also known as pulsed direct current (PDC) electrolysis, the increased number of variables that it introduces to the electrolysis method can change the application of the current to the electrodes and the resulting outcome. This varies from direct current (DC) electrolysis, which only allows the variation of one value, the voltage applied. By utilising conventional pulse width modulation (PMW), multiple dependent variables can be altered, including the type of waveform, typically a rectangular pulse wave, the duty cycle, and the frequency. Currently, there has been a focus on theoretical and experimental research into PDC electrolysis in terms of the electrolysis of water to produce hydrogen. Past research has demonstrated that there is a possibility it can result in a higher electrical efficiency in comparison to DC electrolysis. This would allow electrolysis procedures to produce greater volumes of hydrogen with a reduced electrical energy consumption. Although theoretical research has made large promise for the efficiencies and benefits of utilising pulse electrolysis, it has many contradictions including a common issue that it is difficult to replicate the successes of patents experimentally and produces its own negative effects on the electrolyser.
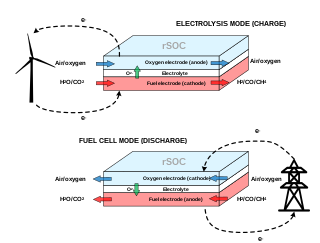
A reversible solid oxide cell (rSOC) is a solid-state electrochemical device that is operated alternatively as a solid oxide fuel cell (SOFC) and a solid oxide electrolysis cell (SOEC). Similarly to SOFCs, rSOCs are made of a dense electrolyte sandwiched between two porous electrodes. Their operating temperature ranges from 600°C to 900°C, hence they benefit from enhanced kinetics of the reactions and increased efficiency with respect to low-temperature electrochemical technologies.
Microbial electrochemical technologies (METs) use microorganisms as electrochemical catalyst, merging the microbial metabolism with electrochemical processes for the production of bioelectricity, biofuels, H2 and other valuable chemicals. Microbial fuel cells (MFC) and microbial electrolysis cells (MEC) are prominent examples of METs. While MFC is used to generate electricity from organic matter typically associated with wastewater treatment, MEC use electricity to drive chemical reactions such as the production of H2 or methane. Recently, microbial electrosynthesis cells (MES) have also emerged as a promising MET, where valuable chemicals can be produced in the cathode compartment. Other MET applications include microbial remediation cell, microbial desalination cell, microbial solar cell, microbial chemical cell, etc.,.








