
A microarray is a multiplex lab-on-a-chip. Its purpose is to simultaneously detect the expression of thousands of biological interactions. It is a two-dimensional array on a solid substrate—usually a glass slide or silicon thin-film cell—that assays (tests) large amounts of biological material using high-throughput screening miniaturized, multiplexed and parallel processing and detection methods. The concept and methodology of microarrays was first introduced and illustrated in antibody microarrays by Tse Wen Chang in 1983 in a scientific publication and a series of patents. The "gene chip" industry started to grow significantly after the 1995 Science Magazine article by the Ron Davis and Pat Brown labs at Stanford University. With the establishment of companies, such as Affymetrix, Agilent, Applied Microarrays, Arrayjet, Illumina, and others, the technology of DNA microarrays has become the most sophisticated and the most widely used, while the use of protein, peptide and carbohydrate microarrays is expanding.
A lab-on-a-chip (LOC) is a device that integrates one or several laboratory functions on a single integrated circuit of only millimeters to a few square centimeters to achieve automation and high-throughput screening. LOCs can handle extremely small fluid volumes down to less than pico-liters. Lab-on-a-chip devices are a subset of microelectromechanical systems (MEMS) devices and sometimes called "micro total analysis systems" (μTAS). LOCs may use microfluidics, the physics, manipulation and study of minute amounts of fluids. However, strictly regarded "lab-on-a-chip" indicates generally the scaling of single or multiple lab processes down to chip-format, whereas "μTAS" is dedicated to the integration of the total sequence of lab processes to perform chemical analysis.
Mechanotaxis refers to the directed movement of cell motility via mechanical cues. In response to fluidic shear stress, for example, cells have been shown to migrate in the direction of the fluid flow. Mechanotaxis is critical in many normal biological processes in animals, such as gastrulation, inflammation, and repair in response to a wound, as well as in mechanisms of diseases such as tumor metastasis.
Cell culture or tissue culture is the process by which cells are grown under controlled conditions, generally outside of their natural environment. After cells of interest have been isolated from living tissue, they can subsequently be maintained under carefully controlled conditions. They need to be kept at body temperature (37 °C) in an incubator. These conditions vary for each cell type, but generally consist of a suitable vessel with a substrate or rich medium that supplies the essential nutrients (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones, and gases (CO2, O2), and regulates the physio-chemical environment (pH buffer, osmotic pressure, temperature). Most cells require a surface or an artificial substrate to form an adherent culture as a monolayer (one single-cell thick), whereas others can be grown free floating in a medium as a suspension culture. This is typically facilitated via use of a liquid, semi-solid, or solid growth medium, such as broth or agar. Tissue culture commonly refers to the culture of animal cells and tissues, with the more specific term plant tissue culture being used for plants. The lifespan of most cells is genetically determined, but some cell-culturing cells have been 'transformed' into immortal cells which will reproduce indefinitely if the optimal conditions are provided.

Dip pen nanolithography (DPN) is a scanning probe lithography technique where an atomic force microscope (AFM) tip is used to directly create patterns on a substrate. It can be done on a range of substances with a variety of inks. A common example of this technique is exemplified by the use of alkane thiolates to imprint onto a gold surface. This technique allows surface patterning on scales of under 100 nanometers. DPN is the nanotechnology analog of the dip pen, where the tip of an atomic force microscope cantilever acts as a "pen", which is coated with a chemical compound or mixture acting as an "ink", and put in contact with a substrate, the "paper".
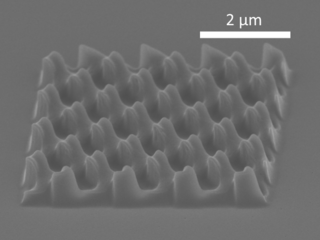
Nanoimprint lithography (NIL) is a method of fabricating nanometer-scale patterns. It is a simple nanolithography process with low cost, high throughput and high resolution. It creates patterns by mechanical deformation of imprint resist and subsequent processes. The imprint resist is typically a monomer or polymer formulation that is cured by heat or UV light during the imprinting. Adhesion between the resist and the template is controlled to allow proper release.

Plasma cleaning is the removal of impurities and contaminants from surfaces through the use of an energetic plasma or dielectric barrier discharge (DBD) plasma created from gaseous species. Gases such as argon and oxygen, as well as mixtures such as air and hydrogen/nitrogen are used. The plasma is created by using high frequency voltages to ionise the low pressure gas, although atmospheric pressure plasmas are now also common.
A nerve guidance conduit is an artificial means of guiding axonal regrowth to facilitate nerve regeneration and is one of several clinical treatments for nerve injuries. When direct suturing of the two stumps of a severed nerve cannot be accomplished without tension, the standard clinical treatment for peripheral nerve injuries is autologous nerve grafting. Due to the limited availability of donor tissue and functional recovery in autologous nerve grafting, neural tissue engineering research has focused on the development of bioartificial nerve guidance conduits as an alternative treatment, especially for large defects. Similar techniques are also being explored for nerve repair in the spinal cord but nerve regeneration in the central nervous system poses a greater challenge because its axons do not regenerate appreciably in their native environment.
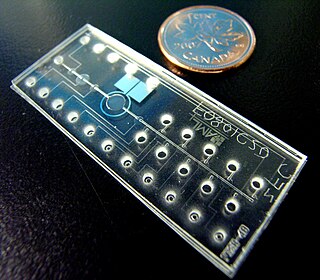
Bio-MEMS is an abbreviation for biomedical microelectromechanical systems. Bio-MEMS have considerable overlap, and is sometimes considered synonymous, with lab-on-a-chip (LOC) and micro total analysis systems (μTAS). Bio-MEMS is typically more focused on mechanical parts and microfabrication technologies made suitable for biological applications. On the other hand, lab-on-a-chip is concerned with miniaturization and integration of laboratory processes and experiments into single chips. In this definition, lab-on-a-chip devices do not strictly have biological applications, although most do or are amenable to be adapted for biological purposes. Similarly, micro total analysis systems may not have biological applications in mind, and are usually dedicated to chemical analysis. A broad definition for bio-MEMS can be used to refer to the science and technology of operating at the microscale for biological and biomedical applications, which may or may not include any electronic or mechanical functions. The interdisciplinary nature of bio-MEMS combines material sciences, clinical sciences, medicine, surgery, electrical engineering, mechanical engineering, optical engineering, chemical engineering, and biomedical engineering. Some of its major applications include genomics, proteomics, molecular diagnostics, point-of-care diagnostics, tissue engineering, single cell analysis and implantable microdevices.

Lung-on-a-chip (LoC), also known as Lung Chips, are micro- and millifluidic organ-on-a-chip devices designed to replicate the structure and function of the human lung, mimicking the breathing motions and fluid dynamics that occur during inhalation and exhalation. LoCs represent the most promising alternative to replace animal testing.
Adsorption is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without surface penetration occurring. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and as such they adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.

Biomaterials are materials that are used in contact with biological systems. Biocompatibility and applicability of surface modification with current uses of metallic, polymeric and ceramic biomaterials allow alteration of properties to enhance performance in a biological environment while retaining bulk properties of the desired device.
An organ-on-a-chip (OOC) is a multi-channel 3-D microfluidic cell culture, integrated circuit (chip) that simulates the activities, mechanics and physiological response of an entire organ or an organ system. It constitutes the subject matter of significant biomedical engineering research, more precisely in bio-MEMS. The convergence of labs-on-chips (LOCs) and cell biology has permitted the study of human physiology in an organ-specific context. By acting as a more sophisticated in vitro approximation of complex tissues than standard cell culture, they provide the potential as an alternative to animal models for drug development and toxin testing.
Microfluidics in chemical biology is the application of microfluidics in the study of chemical biology.
Microfluidic cell culture integrates knowledge from biology, biochemistry, engineering, and physics to develop devices and techniques for culturing, maintaining, analyzing, and experimenting with cells at the microscale. It merges microfluidics, a set of technologies used for the manipulation of small fluid volumes within artificially fabricated microsystems, and cell culture, which involves the maintenance and growth of cells in a controlled laboratory environment. Microfluidics has been used for cell biology studies as the dimensions of the microfluidic channels are well suited for the physical scale of cells. For example, eukaryotic cells have linear dimensions between 10 and 100 μm which falls within the range of microfluidic dimensions. A key component of microfluidic cell culture is being able to mimic the cell microenvironment which includes soluble factors that regulate cell structure, function, behavior, and growth. Another important component for the devices is the ability to produce stable gradients that are present in vivo as these gradients play a significant role in understanding chemotactic, durotactic, and haptotactic effects on cells.
Contact guidance refers to a phenomenon for which the orientation of cells and stress fibers is influenced by geometrical patterns such as nano/microgrooves on substrates, or collagen fibers in gels and soft tissues. This phenomenon was discovered in 1912, and the terminology was introduced in 1945, but it is with the development of tissue engineering that researchers drew increasing attention on this topic, seeing the potential of contact guidance in influencing the morphology and organization of cells. Nevertheless, the biological processes underlying contact guidance are still unclear.

Alvéole is a French company based in Paris and founded in 2010 by Quattrocento, a business accelerator company in the life science field, in collaboration with researchers from the French National Center for Scientific Research with expertise in bioengineering and cell imaging.
Microfluidics refers to the flow of fluid in channels or networks with at least one dimension on the micron scale. In open microfluidics, also referred to as open surface microfluidics or open-space microfluidics, at least one boundary confining the fluid flow of a system is removed, exposing the fluid to air or another interface such as a second fluid.
Open microfluidics can be employed in the multidimensional culturing of cell types for various applications including organ-on-a-chip studies, oxygen-driven reactions, neurodegeneration, cell migration, and other cellular pathways.
Milan Mrksich is an American chemist. He is the Henry Wade Rogers Professor at Northwestern University with appointments in chemistry, biomedical engineering and cell & developmental biology. He also served as both the founding director of the Center for Synthetic Biology and as an associate director of the Robert H. Lurie Comprehensive Cancer Center at Northwestern. Mrksich also served as the Vice President for Research of Northwestern University.









