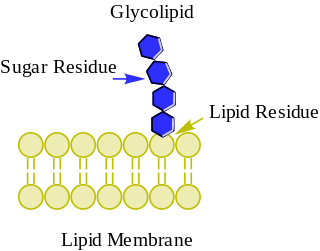
Glycolipids are lipids with a carbohydrate attached by a glycosidic (covalent) bond. Their role is to maintain the stability of the cell membrane and to facilitate cellular recognition, which is crucial to the immune response and in the connections that allow cells to connect to one another to form tissues. Glycolipids are found on the surface of all eukaryotic cell membranes, where they extend from the phospholipid bilayer into the extracellular environment.
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed mainly by cells of the innate immune system, such as dendritic cells, macrophages, monocytes, neutrophils, as well as by epithelial cells, to identify two classes of molecules: pathogen-associated molecular patterns (PAMPs), which are associated with microbial pathogens, and damage-associated molecular patterns (DAMPs), which are associated with components of host's cells that are released during cell damage or death. They are also called primitive pattern recognition receptors because they evolved before other parts of the immune system, particularly before adaptive immunity. PRRs also mediate the initiation of antigen-specific adaptive immune response and release of inflammatory cytokines.

DC-SIGN also known as CD209 is a protein which in humans is encoded by the CD209 gene.

CD23, also known as Fc epsilon RII, or FcεRII, is the "low-affinity" receptor for IgE, an antibody isotype involved in allergy and resistance to parasites, and is important in regulation of IgE levels. Unlike many of the antibody receptors, CD23 is a C-type lectin. It is found on mature B cells, activated macrophages, eosinophils, follicular dendritic cells, and platelets.
Collectins (collagen-containing C-type lectins) are a part of the innate immune system. They form a family of collagenous Ca2+-dependent defense lectins, which are found in animals. Collectins are soluble pattern recognition receptors (PRRs). Their function is to bind to oligosaccharide structure or lipids that are on the surface of microorganisms. Like other PRRs they bind pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) of oligosaccharide origin. Binding of collectins to microorganisms may trigger elimination of microorganisms by aggregation, complement activation, opsonization, activation of phagocytosis, or inhibition of microbial growth. Other functions of collectins are modulation of inflammatory, allergic responses, adaptive immune system and clearance of apoptotic cells.
Siglecs(Sialic acid-binding immunoglobulin-type lectins) are cell surface proteins that bind sialic acid. They are found primarily on the surface of immune cells and are a subset of the I-type lectins. There are 14 different mammalian Siglecs, providing an array of different functions based on cell surface receptor-ligand interactions.

CD94, also known as killer cell lectin-like receptor subfamily D, member 1 (KLRD1) is a human gene.
The mannose receptor is a C-type lectin primarily present on the surface of macrophages, immature dendritic cells and liver sinusoidal endothelial cells, but is also expressed on the surface of skin cells such as human dermal fibroblasts and keratinocytes. It is the first member of a family of endocytic receptors that includes Endo180 (CD280), M-type PLA2R, and DEC-205 (CD205).

Interleukin 6 receptor (IL6R) also known as CD126 is a type I cytokine receptor.

Oxidized low-density lipoprotein receptor 1 also known as lectin-type oxidized LDL receptor 1 (LOX-1) is a protein that in humans is encoded by the OLR1 gene.

CD69 is a human transmembrane C-Type lectin protein encoded by the CD69 gene. It is an early activation marker that is expressed in hematopoietic stem cells, T cells, and many other cell types in the immune system. It is also implicated in T cell differentiation as well as lymphocyte retention in lymphoid organs.

C-type lectin domain family 7 member A or Dectin-1 is a protein that in humans is encoded by the CLEC7A gene. CLEC7A is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded glycoprotein is a small type II membrane receptor with an extracellular C-type lectin-like domain fold and a cytoplasmic domain with a partial immunoreceptor tyrosine-based activation motif. It functions as a pattern-recognition receptor for a variety of β-1,3-linked and β-1,6-linked glucans from fungi and plants, and in this way plays a role in innate immune response. Expression is found on myeloid dendritic cells, monocytes, macrophages and B cells. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. This gene is closely linked to other CTL/CTLD superfamily members on chromosome 12p13 in the natural killer gene complex region.

NKG2-F type II integral membrane protein is a protein that in humans is encoded by the KLRC4 gene.

Killer cell lectin-like receptor subfamily B, member 1, also known as KLRB1, NKR-P1A or CD161, is a human gene.

NKG2-C type II integral membrane protein or NKG2C is a protein that in humans is encoded by the KLRC2 gene. It is also known as or cluster of differentiation 159c (CD159c).

Cord factor, or trehalose dimycolate (TDM), is a glycolipid molecule found in the cell wall of Mycobacterium tuberculosis and similar species. It is the primary lipid found on the exterior of M. tuberculosis cells. Cord factor influences the arrangement of M. tuberculosis cells into long and slender formations, giving its name. Cord factor is virulent towards mammalian cells and critical for survival of M. tuberculosis in hosts, but not outside of hosts. Cord factor has been observed to influence immune responses, induce the formation of granulomas, and inhibit tumor growth. The antimycobacterial drug SQ109 is thought to inhibit TDM production levels and in this way disrupts its cell wall assembly.

NKG2D is an activating receptor (transmembrane protein) belonging to the NKG2 family of C-type lectin-like receptors. NKG2D is encoded by KLRK1 (killer cell lectin like receptor K1) gene which is located in the NK-gene complex (NKC) situated on chromosome 6 in mice and chromosome 12 in humans. In mice, it is expressed by NK cells, NK1.1+ T cells, γδ T cells, activated CD8+ αβ T cells and activated macrophages. In humans, it is expressed by NK cells, γδ T cells and CD8+ αβ T cells. NKG2D recognizes induced-self proteins from MIC and RAET1/ULBP families which appear on the surface of stressed, malignant transformed, and infected cells.

Stimulator of interferon genes (STING), also known as transmembrane protein 173 (TMEM173) and MPYS/MITA/ERIS is a protein that in humans is encoded by the STING1 gene.

Dectin-2 or C-type lectin domain containing 6A is a protein that in humans is encoded by the CLEC6A gene. Dectin-2 is a member of the C-type lectin/C-type lectin-like domain (CTL/CTLD) superfamily. The encoded protein is a type II transmembrane protein with an extracellular carbohydrate recognition domain. It functions as a pattern recognition receptor recognizing α-mannans and as such plays an important role in innate immune response to fungi. Expression is found on macrophages and dendritic cells. It can also be found at low levels in Langerhans cells and peripheral blood monocytes, where expression levels could be increased upon induction of inflammation.

Paired receptors are pairs or clusters of receptor proteins that bind to extracellular ligands but have opposing activating and inhibitory signaling effects. Traditionally, paired receptors are defined as homologous pairs with similar extracellular domains and different cytoplasmic regions, whose genes are located together in the genome as part of the same gene cluster and which evolved through gene duplication. Homologous paired receptors often, but not always, have a shared ligand in common. More broadly, pairs of receptors have been identified that exhibit paired functional behavior - responding to a shared ligand with opposing intracellular signals - but are not closely homologous or co-located in the genome. Paired receptors are highly expressed in the cells of the immune system, especially natural killer (NK) and myeloid cells, and are involved in immune regulation.











