
A retrovirus is a type of virus that inserts a copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. Once inside the host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus.

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development.
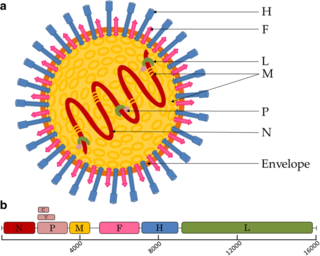
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, and 78 species, three genera of which are unassigned to a subfamily.

The lytic cycle is one of the two cycles of viral reproduction, the other being the lysogenic cycle. The lytic cycle results in the destruction of the infected cell and its membrane. Bacteriophages that only use the lytic cycle are called virulent phages.

Viral replication is the formation of biological viruses during the infection process in the target host cells. Viruses must first get into the cell before viral replication can occur. Through the generation of abundant copies of its genome and packaging these copies, the virus continues infecting new hosts. Replication between viruses is greatly varied and depends on the type of genes involved in them. Most DNA viruses assemble in the nucleus while most RNA viruses develop solely in cytoplasm.

Φ6 is the best-studied bacteriophage of the virus family Cystoviridae. It infects Pseudomonas bacteria. It has a three-part, segmented, double-stranded RNA genome, totalling ~13.5 kb in length. Φ6 and its relatives have a lipid membrane around their nucleocapsid, a rare trait among bacteriophages. It is a lytic phage, though under certain circumstances has been observed to display a delay in lysis which may be described as a "carrier state".
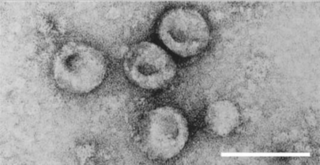
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.
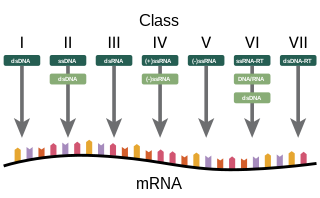
Baltimore classification is a system used to classify viruses based on their manner of messenger RNA (mRNA) synthesis. By organizing viruses based on their manner of mRNA production, it is possible to study viruses that behave similarly as a distinct group. Seven Baltimore groups are described that take into consideration whether the viral genome is made of deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), whether the genome is single- or double-stranded, and whether the sense of a single-stranded RNA genome is positive or negative.
The murine leukemia viruses are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.

Molluscum contagiosum virus (MCV) is a DNA poxvirus that causes the human skin infection molluscum contagiosum. Molluscum contagiosum affects about 200,000 people a year, about 1% of all diagnosed skin diseases. Diagnosis is based on the size and shape of the skin lesions and can be confirmed with a biopsy, as the virus cannot be routinely cultured. Molluscum contagiosum virus is the only species in the genus Molluscipoxvirus. MCV is a member of the subfamily Chordopoxvirinae of family Poxviridae. Other commonly known viruses that reside in the subfamily Chordopoxvirinae are variola virus and monkeypox virus.
Phycodnaviridae is a family of large (100–560 kb) double-stranded DNA viruses that infect marine or freshwater eukaryotic algae. Viruses within this family have a similar morphology, with an icosahedral capsid. As of 2014, there were 33 species in this family, divided among 6 genera. This family belongs to a super-group of large viruses known as nucleocytoplasmic large DNA viruses. Evidence was published in 2014 suggesting that specific strains of Phycodnaviridae might infect humans rather than just algal species, as was previously believed. Most genera under this family enter the host cell by cell receptor endocytosis and replicate in the nucleus. Phycodnaviridae play important ecological roles by regulating the growth and productivity of their algal hosts. Algal species such Heterosigma akashiwo and the genus Chrysochromulina can form dense blooms which can be damaging to fisheries, resulting in losses in the aquaculture industry. Heterosigma akashiwo virus (HaV) has been suggested for use as a microbial agent to prevent the recurrence of toxic red tides produced by this algal species. Phycodnaviridae cause death and lysis of freshwater and marine algal species, liberating organic carbon, nitrogen and phosphorus into the water, providing nutrients for the microbial loop.

Double-stranded RNA viruses are a polyphyletic group of viruses that have double-stranded genomes made of ribonucleic acid. The double-stranded genome is used to transcribe a positive-strand RNA by the viral RNA-dependent RNA polymerase (RdRp). The positive-strand RNA may be used as messenger RNA (mRNA) which can be translated into viral proteins by the host cell's ribosomes. The positive-strand RNA can also be replicated by the RdRp to create a new double-stranded viral genome.

A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Since Dmitri Ivanovsky's 1892 article describing a non-bacterial pathogen infecting tobacco plants and the discovery of the tobacco mosaic virus by Martinus Beijerinck in 1898, more than 9,000 virus species have been described in detail of the millions of types of viruses in the environment. Viruses are found in almost every ecosystem on Earth and are the most numerous type of biological entity. The study of viruses is known as virology, a subspeciality of microbiology.
Plectrovirus is a genus of viruses, in the family Plectroviridae. Bacteria in the phylum Tenericutes serve as natural hosts, making these viruses bacteriophages. Acholeplasma virus L51 is the only species in the genus.
Adenovirus genomes are linear, non-segmented double-stranded (ds) DNA molecules that are typically 26-46 Kbp long, containing 23-46 protein-coding genes. The example used for the following description is Human adenovirus E, a mastadenovirus with a 36 Kbp genome containing 38 protein-coding genes. While the precise number and identity of genes varies among adenoviruses, the basic principles of genome organization and the functions of most of the genes described in this article are shared among all adenoviruses.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.
This glossary of virology is a list of definitions of terms and concepts used in virology, the study of viruses, particularly in the description of viruses and their actions. Related fields include microbiology, molecular biology, and genetics.

Positive-strand RNA viruses are a group of related viruses that have positive-sense, single-stranded genomes made of ribonucleic acid. The positive-sense genome can act as messenger RNA (mRNA) and can be directly translated into viral proteins by the host cell's ribosomes. Positive-strand RNA viruses encode an RNA-dependent RNA polymerase (RdRp) which is used during replication of the genome to synthesize a negative-sense antigenome that is then used as a template to create a new positive-sense viral genome.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.

The woolly monkey hepatitis B virus (WMHBV) is a viral species of the Orthohepadnavirus genus of the Hepadnaviridae family. Its natural host is the woolly monkey (Lagothrix), an inhabitant of South America categorized as a New World primate. WMHBV, like other hepatitis viruses, infects the hepatocytes, or liver cells, of its host organism. It can cause hepatitis, liver necrosis, cirrhosis, and hepatocellular carcinoma. Because nearly all species of Lagothrix are threatened or endangered, researching and developing a vaccine and/or treatment for WMHBV is important for the protection of the whole woolly monkey genus.

















