
Novartis International AG is a Swiss multinational pharmaceutical corporation based in Basel, Switzerland. It is one of the largest pharmaceutical companies in the world.

A medication is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management.
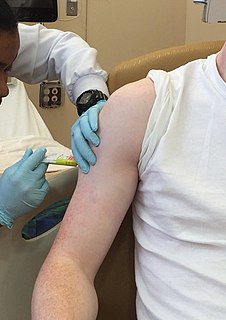
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

The American Medical Association (AMA) is a professional association and lobbying group of physicians and medical students. Founded in 1847, it is headquartered in Chicago, Illinois. Membership was approximately 240,000 in 2016.

The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients, with the aim to cure them, vaccinate them, or alleviate symptoms. Pharmaceutical companies may deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations that govern the patenting, testing, safety, efficacy using drug testing and marketing of drugs. The global pharmaceuticals market produced treatments worth $1,228.45 billion in 2020 and showed a compound annual growth rate (CAGR) of 1.8%.

Vertex Pharmaceuticals is an American biopharmaceutical company based in Boston, Massachusetts. It was one of the first biotech firms to use an explicit strategy of rational drug design rather than combinatorial chemistry. It maintains headquarters in South Boston, Massachusetts, and three research facilities, in San Diego, California, and Milton Park, near Oxford, England.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade.
The Drugs for Neglected Diseases initiative (DNDi) is a collaborative, patients' needs-driven, non-profit drug research and development (R&D) organization that is developing new treatments for neglected diseases, notably leishmaniasis, sleeping sickness, Chagas disease, malaria, filarial diseases, mycetoma, paediatric HIV, and hepatitis C. DNDi's malaria activities were transferred to Medicines for Malaria Venture (MMV) in 2015.
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis is the assessment that a particular [medical] condition is present while an indication is a reason for use. The opposite of an indication is a contraindication, a reason to withhold a certain medical treatment because the risks of treatment clearly outweigh the benefits.

Ronald Hans Anton Plasterk is a Dutch scientist, entrepreneur and retired politician of the Labour Party (PvdA). He has earned a PhD degree in biology, specialised in molecular genetics. Being a former Minister of the Dutch government, he has been the founder and CEO of Frame Cancer Therapeutics since December 2018. Next to his work at Frame, he has been appointed as professor at the University of Amsterdam since September 2018.
Expanded access or compassionate use is the use of an unapproved drug or medical device under special forms of investigational new drug applications (IND) or IDE application for devices, outside of a clinical trial, by people with serious or life-threatening conditions who do not meet the enrollment criteria for the clinical trial in progress.
Ibalizumab, sold under the brand name Trogarzo, is a non-immunosuppressive humanised monoclonal antibody that binds CD4, the primary receptor for HIV, and inhibits HIV from entering cells. It is a post-attachment inhibitor, blocking HIV from binding to the CCR5 and CXCR4 co-receptors after HIV binds to the CD4 receptor on the surface of a CD4 cell. Post-attachment inhibitors are a subclass of HIV drugs called entry inhibitors.
Medication costs, also known as drug costs are a common health care cost for many people and health care systems. Prescription costs are the costs to the end consumer. Medication costs are influenced by multiple factors such as patents, stakeholder influence, and marketing expenses. A number of countries including Canada, parts of Europe, and Brasil use external reference pricing as a means to compare drug prices and to determine a base price for a particular medication. Other countries use pharmacoeconomics, which looks at the cost/benefit of a product in terms of quality of life, alternative treatments, and cost reduction or avoidance in other parts of the health care system. Structures like the UK's National Institute for Health and Clinical Excellence and to a lesser extent Canada's Common Drug Review evaluate products in this way.

Actelion is a pharmaceuticals and biotechnology company established in December 1997, headquartered in Allschwil near Basel, Switzerland.
Alexion Pharmaceuticals, a subsidiary of AstraZeneca, is an American pharmaceutical company headquartered in Boston, Massachusetts that specializes in orphan drugs to treat rare diseases.

Boots UK Limited, trading as Boots, is a British health and beauty retailer and pharmacy chain in the United Kingdom and other countries and territories including Ireland, Italy, Norway, the Netherlands, Thailand and Indonesia.
The Access to Medicine Index is a ranking system published biennially since 2008 by the Access to Medicine Foundation in Amsterdam, the Netherlands, an international not-for-profit organisation, funded by the Dutch Ministry of Foreign Affairs, the UK Foreign, Commonwealth, and Development Office (FCDO), the Bill & Melinda Gates Foundation, Wellcome Trust, and Axa Investment Managers. It ranks the world's 20 largest pharmaceutical companies according to their ability to make their pharmaceutical drugs more available, affordable, accessible and acceptable in 106 low- to middle-income countries. The biennial index aims to stimulate industry to improve access in developing countries, to show the activities of their peers, and allow them, governments, investors, civil society, patient organisations and academia to gather and form a common view of how pharmaceutical companies can make further progress.

Moderna, Inc., is an American pharmaceutical and biotechnology company based in Cambridge, Massachusetts that focuses on RNA therapeutics, primarily mRNA vaccines. These vaccines use a copy of a molecule called messenger RNA (mRNA) to produce an immune response.
RxAll Inc. is an health information technology/big data startup company. The company works with drug regulatory agencies and other stakeholdersto reduce fake medicines and make sure that patients receive high quality verified drugs, and help pharma manufacturers gain more sales, and patients have better access to high quality drugs.

COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID-19). From early 2020 through 2021, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of preclinical or clinical research, with 419 potential COVID-19 drugs in clinical trials, as of April 2021.












