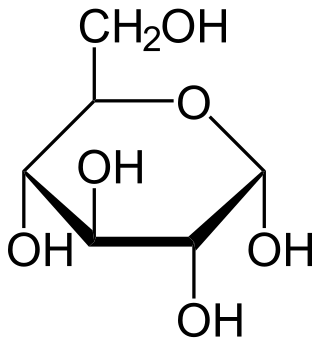
Glucose is a sugar with the molecular formula C6H12O6. Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world.

Protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration.

Glucose transporters are a wide group of membrane proteins that facilitate the transport of glucose across the plasma membrane, a process known as facilitated diffusion. Because glucose is a vital source of energy for all life, these transporters are present in all phyla. The GLUT or SLC2A family are a protein family that is found in most mammalian cells. 14 GLUTS are encoded by human genome. GLUT is a type of uniporter transporter protein.
Glucose transporter type 4 (GLUT4), also known as solute carrier family 2, facilitated glucose transporter member 4, is a protein encoded, in humans, by the SLC2A4 gene. GLUT4 is the insulin-regulated glucose transporter found primarily in adipose tissues and striated muscle. The first evidence for this distinct glucose transport protein was provided by David James in 1988. The gene that encodes GLUT4 was cloned and mapped in 1989.
Glucose transporter 1, also known as solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1), is a uniporter protein that in humans is encoded by the SLC2A1 gene. GLUT1 facilitates the transport of glucose across the plasma membranes of mammalian cells. This gene encodes a facilitative glucose transporter that is highly expressed in erythrocytes and endothelial cells, including cells of the blood–brain barrier. The encoded protein is found primarily in the cell membrane and on the cell surface, where it can also function as a receptor for human T-cell leukemia virus (HTLV) I and II. GLUT1 accounts for 2 percent of the protein in the plasma membrane of erythrocytes. Mutations in this gene can cause GLUT1 deficiency syndrome 1, GLUT1 deficiency syndrome 2, idiopathic generalized epilepsy 12, dystonia 9, and stomatin-deficient cryohydrocytosis.
Glucose transporter 3, also known as solute carrier family 2, facilitated glucose transporter member 3 (SLC2A3) is a protein that in humans is encoded by the SLC2A3 gene. GLUT3 facilitates the transport of glucose across the plasma membranes of mammalian cells. GLUT3 is most known for its specific expression in neurons and has originally been designated as the neuronal GLUT. GLUT3 has been studied in other cell types with specific glucose requirements, including sperm, preimplantation embryos, circulating white blood cells and carcinoma cell lines.
GLUT8 also known as SLC2A8 is the eighth member of glucose transporter superfamily.

Rac1, also known as Ras-related C3 botulinum toxin substrate 1, is a protein found in human cells. It is encoded by the RAC1 gene. This gene can produce a variety of alternatively spliced versions of the Rac1 protein, which appear to carry out different functions.

Solute carrier family 2, facilitated glucose transporter member 9 is a protein that in humans is encoded by the SLC2A9 gene.
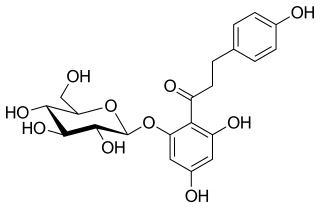
Phlorizin is a glucoside of phloretin, a dihydrochalcone. A white solid, samples often appear yellow owing to impurities. It is of sweet taste and contains four molecules of water in the crystal. Above 200 °C, it decomposes to give rufin. It is poorly soluble in ether and cold water, but soluble in ethanol and hot water. Upon prolonged exposure to aqueous solutions phlorizin hydrolyzes to phloretin and glucose.

mTOR inhibitors are a class of drugs that inhibit the mechanistic target of rapamycin (mTOR), which is a serine/threonine-specific protein kinase that belongs to the family of phosphatidylinositol-3 kinase (PI3K) related kinases (PIKKs). mTOR regulates cellular metabolism, growth, and proliferation by forming and signaling through two protein complexes, mTORC1 and mTORC2. The most established mTOR inhibitors are so-called rapalogs, which have shown tumor responses in clinical trials against various tumor types.

mTORC1, also known as mammalian target of rapamycin complex 1 or mechanistic target of rapamycin complex 1, is a protein complex that functions as a nutrient/energy/redox sensor and controls protein synthesis.
mTOR Complex 2 (mTORC2) is an acutely rapamycin-insensitive protein complex formed by serine/threonine kinase mTOR that regulates cell proliferation and survival, cell migration and cytoskeletal remodeling. The complex itself is rather large, consisting of seven protein subunits. The catalytic mTOR subunit, DEP domain containing mTOR-interacting protein (DEPTOR), mammalian lethal with sec-13 protein 8, and TTI1/TEL2 complex are shared by both mTORC2 and mTORC1. Rapamycin-insensitive companion of mTOR (RICTOR), mammalian stress-activated protein kinase interacting protein 1 (mSIN1), and protein observed with rictor 1 and 2 (Protor1/2) can only be found in mTORC2. Rictor has been shown to be the scaffold protein for substrate binding to mTORC2.
Glucose-6-Phosphate Translocase is an enzyme that in humans is encoded by the SLC37A4 gene. It consists of three subunits, each of which are vital components of the multi-enzyme Glucose-6-Phosphatase Complex (G6Pase). This important enzyme complex is located within the membrane of the endoplasmic reticulum, and catalyzes the terminal reactions in both glycogenolysis and gluconeogenesis. The G6Pase complex is most abundant in liver tissue, but also present in kidney cells, small intestine, pancreatic islets and at a lower concentration in the gallbladder. The G6Pase complex is highly involved in the regulation of homeostasis and blood glucose levels. Within this framework of glucose regulation, the translocase components are responsible for transporting the substrates and products across the endoplasmic reticulum membrane, resulting in the release of free glucose into the bloodstream.
Gliflozins are a class of drugs in the treatment of type 2 diabetes (T2D). They act by inhibiting sodium/glucose cotransporter 2 (SGLT-2), and are therefore also called SGLT-2 inhibitors. The efficacy of the drug is dependent on renal excretion and prevents glucose from going into blood circulation by promoting glucosuria. The mechanism of action is insulin independent.
SGLT2 inhibitors, also called gliflozins or flozins, are a class of medications that modulate sodium-glucose transport proteins in the nephron, unlike SGLT1 inhibitors that perform a similar function in the intestinal mucosa. The foremost metabolic effect of this is to inhibit reabsorption of glucose in the kidney and therefore lower blood sugar. They act by inhibiting sodium-glucose transport protein 2 (SGLT2). SGLT2 inhibitors are used in the treatment of type II diabetes mellitus (T2DM). Apart from blood sugar control, gliflozins have been shown to provide significant cardiovascular benefit in patients with type II diabetes (T2DM). Several medications of this class have been approved or are currently under development. In studies on canagliflozin, a member of this class, the medication was found to enhance blood sugar control as well as reduce body weight and systolic and diastolic blood pressure.
Acyl-protein thioesterases are enzymes that cleave off lipid modifications on proteins, located on the sulfur atom of cysteine residues linked via a thioester bond. Acyl-protein thioesterases are part of the α/β hydrolase superfamily of proteins and have a conserved catalytic triad. For that reason, acyl-protein thioesterases are also able to hydrolyze oxygen-linked ester bonds.
Immunometabolism is a branch of biology that studies the interplay between metabolism and immunology in all organisms. In particular, immunometabolism is the study of the molecular and biochemical underpinninngs for i) the metabolic regulation of immune function, and ii) the regulation of metabolism by molecules and cells of the immune system. Further categorization includes i) systemic immunometabolism and ii) cellular immunometabolism.
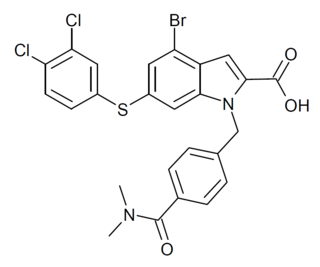
HY-124798 (Rheb inhibitor NR1) is the first compound to be developed that acts as a potent and selective inhibitor of Rheb, a GTP-binding protein which acts as an endogenous activator of the mechanistic target of rapamycin (mTOR) subtype mTORC1. Since many of the side effects of rapamycin and its analogues are thought to result from binding to the other subtype mTORC2, it is hoped that selective inhibition of mTORC1 should have a more selective effects profile. As mTORC1 and mTORC2 have binding sites that are very similar in structure, it has been challenging to develop highly subtype selective inhibitors, making indirect inhibition via modulation of other messenger proteins such as Rheb an attractive approach. However, since HY-124798 has a relatively weak IC50 of 2.1μM, and Rheb also has other targets in addition to mTORC1, it remains to be established whether it will deliver the hoped for improvements in pharmacological profile.
The arrestin family of proteins is subdivided into α-arrestins (also referred to as arrestin-related trafficking adaptors or arrestin-like yeast proteins in yeast or ARRDCs in mammals, β-arrestins and Vps26-like arrestins proteins. The α-Arrestins are an ancestral branch of the larger arrestin family of proteins and they are conserved across eukaryotes but are best characterized in the budding yeast Saccharomyces cerevisiae; to-date there are 6 α-arrestins identified in mammalian cells and 14 α-arrestins identified in the budding yeast Saccharomyces cerevisiae. The yeast α-arrestin family comprises Ldb19/Art1, Ecm21/Art2, Aly1/Art6, Aly2/Art3, Rod1/Art4, Rog3/Art7, Art5, Csr2/Art8, Rim8/Art9, Art10, Bul1, Bul2, Bul3 and Spo23. The best characterized α-arrestin function to date is their endocytic regulation of plasma membrane proteins, including G-protein coupled receptors and nutrient transporters. α-Arrestins control endocytosis of these membrane proteins in response to cellular stressors, including nutrient or metal ion excess.











