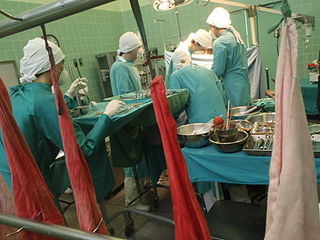
Organ transplantation is a medical procedure in which an organ is removed from one body and placed in the body of a recipient, to replace a damaged or missing organ. The donor and recipient may be at the same location, or organs may be transported from a donor site to another location. Organs and/or tissues that are transplanted within the same person's body are called autografts. Transplants that are recently performed between two subjects of the same species are called allografts. Allografts can either be from a living or cadaveric source.
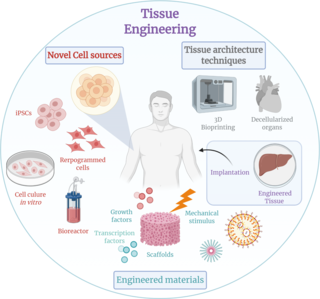
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can is considered as a field of its own.

Transplant rejection occurs when transplanted tissue is rejected by the recipient's immune system, which destroys the transplanted tissue. Transplant rejection can be lessened by determining the molecular similitude between donor and recipient and by use of immunosuppressant drugs after transplant.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.

Xenotransplantation, or heterologous transplant, is the transplantation of living cells, tissues or organs from one species to another. Such cells, tissues or organs are called xenografts or xenotransplants. It is contrasted with allotransplantation, syngeneic transplantation or isotransplantation and autotransplantation. Xenotransplantation is an artificial method of creating an animal-human chimera, that is, a human with a subset of animal cells. In contrast, an individual where each cell contains genetic material from a human and an animal is called a human–animal hybrid.
Regenerative medicine deals with the "process of replacing, engineering or regenerating human or animal cells, tissues or organs to restore or establish normal function". This field holds the promise of engineering damaged tissues and organs by stimulating the body's own repair mechanisms to functionally heal previously irreparable tissues or organs.

Cell therapy is a therapy in which viable cells are injected, grafted or implanted into a patient in order to effectuate a medicinal effect, for example, by transplanting T-cells capable of fighting cancer cells via cell-mediated immunity in the course of immunotherapy, or grafting stem cells to regenerate diseased tissues.

Organ printing utilizes techniques similar to conventional 3D printing where a computer model is fed into a printer that lays down successive layers of plastics or wax until a 3D object is produced. In the case of organ printing, the material being used by the printer is a biocompatible plastic. The biocompatible plastic forms a scaffold that acts as the skeleton for the organ that is being printed. As the plastic is being laid down, it is also seeded with human cells from the patient's organ that is being printed for. After printing, the organ is transferred to an incubation chamber to give the cells time to grow. After a sufficient amount of time, the organ is implanted into the patient.
Neural tissue engineering is a specific sub-field of tissue engineering. Neural tissue engineering is primarily a search for strategies to eliminate inflammation and fibrosis upon implantation of foreign substances. Often foreign substances in the form of grafts and scaffolds are implanted to promote nerve regeneration and to repair damage caused to nerves of both the central nervous system (CNS) and peripheral nervous system (PNS) by an injury.
Transplantable organs and tissues may refer to both organs and tissues that are relatively often transplanted, as well as organs and tissues which are relatively seldom transplanted. In addition to this it may also refer to possible-transplants which are still in the experimental stage.
MIRA is a multidisciplinary and complementary method for treating many chronic diseases. The MIRA Procedure is a result of combining efforts from different medical fields developed in the University of Chicago in 1992. It basically consists in medically grafting live rejuvenated tissue in the form of autologous adipose adult stem cells to a damaged organ in order to restore it and improve its function. This method is currently approved by the U.S. Food and Drug Administration (FDA).

Decellularization is the process used in biomedical engineering to isolate the extracellular matrix (ECM) of a tissue from its inhabiting cells, leaving an ECM scaffold of the original tissue, which can be used in artificial organ and tissue regeneration. Organ and tissue transplantation treat a variety of medical problems, ranging from end organ failure to cosmetic surgery. One of the greatest limitations to organ transplantation derives from organ rejection caused by antibodies of the transplant recipient reacting to donor antigens on cell surfaces within the donor organ. Because of unfavorable immune responses, transplant patients suffer a lifetime taking immunosuppressing medication. Stephen F. Badylak pioneered the process of decellularization at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. This process creates a natural biomaterial to act as a scaffold for cell growth, differentiation and tissue development. By recellularizing an ECM scaffold with a patient’s own cells, the adverse immune response is eliminated. Nowadays, commercially available ECM scaffolds are available for a wide variety of tissue engineering. Using peracetic acid to decellularize ECM scaffolds have been found to be false and only disinfects the tissue.
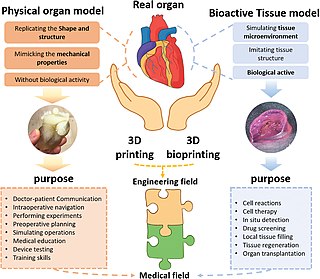
Three dimensional (3D) bioprinting is the utilization of 3D printing–like techniques to combine cells, growth factors, bio-inks, and biomaterials to fabricate functional structures that were traditionally used for tissue engineering applications but in recent times have seen increased interest in other applications such as biosensing, and environmental remediation. Generally, 3D bioprinting utilizes a layer-by-layer method to deposit materials known as bio-inks to create tissue-like structures that are later used in various medical and tissue engineering fields. 3D bioprinting covers a broad range of bioprinting techniques and biomaterials. Currently, bioprinting can be used to print tissue and organ models to help research drugs and potential treatments. Nonetheless, translation of bioprinted living cellular constructs into clinical application is met with several issues due to the complexity and cell number necessary to create functional organs. However, innovations span from bioprinting of extracellular matrix to mixing cells with hydrogels deposited layer by layer to produce the desired tissue. In addition, 3D bioprinting has begun to incorporate the printing of scaffolds which can be used to regenerate joints and ligaments. Apart from these, 3D bioprinting has recently been used in environmental remediation applications, including the fabrication of functional biofilms that host functional microorganisms that can facilitate pollutant removal.
A bioartificial heart is an engineered heart that contains the extracellular structure of a decellularized heart and cellular components from a different source. Such hearts are of particular interest for therapy as well as research into heart disease. The first bioartificial hearts were created in 2008 using cadaveric rat hearts. In 2014, human-sized bioartificial pig hearts were constructed. Bioartificial hearts have not been developed yet for clinical use, although the recellularization of porcine hearts with human cells opens the door to xenotransplantation.
Regeneration in humans is the regrowth of lost tissues or organs in response to injury. This is in contrast to wound healing, or partial regeneration, which involves closing up the injury site with some gradation of scar tissue. Some tissues such as skin, the vas deferens, and large organs including the liver can regrow quite readily, while others have been thought to have little or no capacity for regeneration following an injury.

Limbal stem cells, also known as corneal epithelial stem cells, are unipotent stem cells located in the basal epithelial layer of the corneal limbus. They form the border between the cornea and the sclera. Characteristics of limbal stem cells include a slow turnover rate, high proliferative potential, clonogenicity, expression of stem cell markers, as well as the ability to regenerate the entire corneal epithelium. Limbal stem cell proliferation has the role of maintaining the cornea; for example, by replacing cells that are lost via tears. Additionally, these cells also prevent the conjunctival epithelial cells from migrating onto the surface of the cornea.
Bio-inks are materials used to produce engineered/artificial live tissue using 3D printing. These inks are mostly composed of the cells that are being used, but are often used in tandem with additional materials that envelope the cells. The combination of cells and usually biopolymer gels are defined as a bio-ink. They must meet certain characteristics, including such as rheological, mechanical, biofunctional and biocompatibility properties, among others. Using bio-inks provides a high reproducibility and precise control over the fabricated constructs in an automated manner. These inks are considered as one of the most advanced tools for tissue engineering and regenerative medicine (TERM).

An artificial ovary is a potential fertility preservation treatment that aims to mimic the function of the natural ovary.

Microgravity bioprinting is the utilization of 3D bioprinting techniques under microgravity conditions to fabricate highly complex, functional tissue and organ structures. The zero gravity environment circumvents some of the current limitations of bioprinting on Earth including magnetic field disruption and biostructure retention during the printing process. Microgravity bioprinting is one of the initial steps to advancing in space exploration and colonization while furthering the possibilities of regenerative medicine.
Tissue transplantation is a surgical procedure involving the removal of tissue from a donor site or the creation of new tissue, followed by tissue transfer to the recipient site. The aim of tissue transplantation is to repair or replace tissues that are missing, damaged, or diseased, thereby improving patients’ survival, functionality and quality of life.














