Related Research Articles
Teratology is the study of abnormalities of physiological development in organisms during their life span. It is a sub-discipline in medical genetics which focuses on the classification of congenital abnormalities in dysmorphology caused by teratogens. Teratogens are substances that may cause non-heritable birth defects via a toxic effect on an embryo or fetus. Defects include malformations, disruptions, deformations, and dysplasia that may cause stunted growth, delayed mental development, or other congenital disorders that lack structural malformations. The related term developmental toxicity includes all manifestations of abnormal development that are caused by environmental insult. The extent to which teratogens will impact an embryo is dependent on several factors, such as how long the embryo has been exposed, the stage of development the embryo was in when exposed, the genetic makeup of the embryo, and the transfer rate of the teratogen.

Microcephaly is a medical condition involving a smaller-than-normal head. Microcephaly may be present at birth or it may develop in the first few years of life. Brain development is often affected; people with this disorder often have an intellectual disability, poor motor function, poor speech, abnormal facial features, seizures and dwarfism.

A birth defect is an abnormal condition that is present at birth, regardless of its cause. Birth defects may result in disabilities that may be physical, intellectual, or developmental. The disabilities can range from mild to severe. Birth defects are divided into two main types: structural disorders in which problems are seen with the shape of a body part and functional disorders in which problems exist with how a body part works. Functional disorders include metabolic and degenerative disorders. Some birth defects include both structural and functional disorders.

Waardenburg syndrome is a group of rare genetic conditions characterised by at least some degree of congenital hearing loss and pigmentation deficiencies, which can include bright blue eyes, a white forelock or patches of light skin. These basic features constitute type 2 of the condition; in type 1, there is also a wider gap between the inner corners of the eyes called telecanthus, or dystopia canthorum. In type 3, which is rare, the arms and hands are also malformed, with permanent finger contractures or fused fingers, while in type 4, the person also has Hirschsprung's disease. There also exist at least two types that can result in central nervous system (CNS) symptoms such as developmental delay and muscle tone abnormalities.

A congenital heart defect (CHD), also known as a congenital heart anomaly, congenital cardiovascular malformation, and congenital heart disease, is a defect in the structure of the heart or great vessels that is present at birth. A congenital heart defect is classed as a cardiovascular disease. Signs and symptoms depend on the specific type of defect. Symptoms can vary from none to life-threatening. When present, symptoms are variable and may include rapid breathing, bluish skin (cyanosis), poor weight gain, and feeling tired. CHD does not cause chest pain. Most congenital heart defects are not associated with other diseases. A complication of CHD is heart failure.

The neural crest is a ridge-like structure that is formed transiently between the epidermal ectoderm and neural plate during vertebrate development. Neural crest cells originate from this structure through the epithelial-mesenchymal transition, and in turn give rise to a diverse cell lineage—including melanocytes, craniofacial cartilage and bone, smooth muscle, dentin, peripheral and enteric neurons, adrenal medulla and glia.

In neuroanatomy, a gyrus is a ridge on the cerebral cortex. It is generally surrounded by one or more sulci. Gyri and sulci create the folded appearance of the brain in humans and other mammals.

Persistent truncus arteriosus (PTA), often referred to simply as truncus arteriosus, is a rare form of congenital heart disease that presents at birth. In this condition, the embryological structure known as the truncus arteriosus fails to properly divide into the pulmonary trunk and aorta. This results in one arterial trunk arising from the heart and providing mixed blood to the coronary arteries, pulmonary arteries, and systemic circulation. For the International Classification of Diseases (ICD-11), the International Paediatric and Congenital Cardiac Code (IPCCC) was developed to standardize the nomenclature of congenital heart disease. Under this system, English is now the official language, and persistent truncus arteriosus should properly be termed common arterial trunk.

Dandy–Walker malformation (DWM), also known as Dandy–Walker syndrome (DWS), is a rare congenital brain malformation in which the part joining the two hemispheres of the cerebellum does not fully form, and the fourth ventricle and space behind the cerebellum are enlarged with cerebrospinal fluid. Most of those affected develop hydrocephalus within the first year of life, which can present as increasing head size, vomiting, excessive sleepiness, irritability, downward deviation of the eyes and seizures. Other, less common symptoms are generally associated with comorbid genetic conditions and can include congenital heart defects, eye abnormalities, intellectual disability, congenital tumours, other brain defects such as agenesis of the corpus callosum, skeletal abnormalities, an occipital encephalocele or underdeveloped genitalia or kidneys. It is sometimes discovered in adolescents or adults due to mental health problems.

Zinc finger protein GLI2 also known as GLI family zinc finger 2 is a protein that in humans is encoded by the GLI2 gene. The protein encoded by this gene is a transcription factor.

The neural fold is a structure that arises during neurulation in the embryonic development of both birds and mammals among other organisms. This structure is associated with primary neurulation, meaning that it forms by the coming together of tissue layers, rather than a clustering, and subsequent hollowing out, of individual cells. In humans, the neural folds are responsible for the formation of the anterior end of the neural tube. The neural folds are derived from the neural plate, a preliminary structure consisting of elongated ectoderm cells. The folds give rise to neural crest cells, as well as bringing about the formation of the neural tube.

Mesenchyme is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.
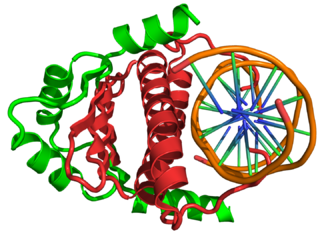
In the field of molecular biology, myocyte enhancer factor-2 (Mef2) proteins are a family of transcription factors which through control of gene expression are important regulators of cellular differentiation and consequently play a critical role in embryonic development. In adult organisms, Mef2 proteins mediate the stress response in some tissues. Mef2 proteins contain both MADS-box and Mef2 DNA-binding domains.
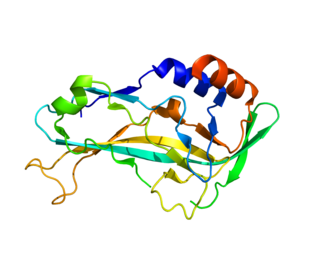
T-box transcription factor TBX5, is a protein that in humans is encoded by the TBX5 gene. Abnormalities in the TBX5 gene can result in altered limb development, Holt-Oram syndrome, Tetra-amelia syndrome, and cardiac and skeletal problems.

Heart- and neural crest derivatives-expressed protein 1 is a protein that in humans is encoded by the HAND1 gene.

Homeobox protein goosecoid(GSC) is a homeobox protein that is encoded in humans by the GSC gene. Like other homeobox proteins, goosecoid functions as a transcription factor involved in morphogenesis. In Xenopus, GSC is thought to play a crucial role in the phenomenon of the Spemann-Mangold organizer. Through lineage tracing and timelapse microscopy, the effects of GSC on neighboring cell fates could be observed. In an experiment that injected cells with GSC and observed the effects of uninjected cells, GSC recruited neighboring uninjected cells in the dorsal blastopore lip of the Xenopus gastrula to form a twinned dorsal axis, suggesting that the goosecoid protein plays a role in the regulation and migration of cells during gastrulation.

Fibrochondrogenesis is a rare autosomal recessive form of osteochondrodysplasia, causing abnormal fibrous development of cartilage and related tissues.

Sclerocornea is an extremely rare congenital anomaly of the eye, it is considered a form of congenital corneal opacity (CCO) with no clear gender bias, in which the cornea blends with sclera, having no clear-cut boundary. The extent of the resulting opacity varies from peripheral to total. While the exact historical origins of its documentation are unclear, studies on sclerocornea has long been recognized in ophthalmology as a rare but significant anomaly going as far back as the 1960's. The severe form is thought to be inherited in an autosomal recessive manner, but there may be another, milder form that is expressed in a dominant fashion. In some cases the patients also have abnormalities beyond the eye (systemic), such as limb deformities and craniofacial and genitourinary defects.
Neural crest cells are multipotent cells required for the development of cells, tissues and organ systems. A subpopulation of neural crest cells are the cardiac neural crest complex. This complex refers to the cells found amongst the midotic placode and somite 3 destined to undergo epithelial-mesenchymal transformation and migration to the heart via pharyngeal arches 3, 4 and 6.

Grainyhead-like genes are a family of highly conserved transcription factors that are functionally and structurally homologous across a large number of vertebrate and invertebrate species. For an estimated 100 million years or more, this genetic family has been evolving alongside life to fine tune the regulation of epithelial barrier integrity during development, fine-tuning epithelial barrier establishment, maintenance and subsequent homeostasis. The three main orthologues, Grainyhead-like 1, 2 and 3, regulate numerous genetic pathways within different organisms and perform analogous roles between them, ranging from neural tube closure, wound healing, establishment of the craniofacial skeleton and repair of the epithelium. When Grainyhead-like genes are impaired, due to genetic mutations in embryogenesis, it will cause the organism to present with developmental defects that largely affect ectodermal tissues in which they are expressed. These subsequent congenital disorders, including cleft lip and exencephaly, vary greatly in their severity and impact on the quality of life for the affected individual. There is much still to learn about the function of these genes and the more complex roles of Grainyhead-like genes are yet to be discovered.
References
- ↑ Etchevers, Heather C.; Amiel, Jeanne; Lyonnet, Stanislas (2006). "Molecular Bases of Human Neurocristopathies". Neural Crest Induction and Differentiation, Volume 589 . Advances in Experimental Medicine and Biology. Vol. 589. pp. 213–34. doi:10.1007/978-0-387-46954-6_14. ISBN 978-0-387-35136-0. PMID 17076285.
- ↑ Vega-Lopez, Guillermo A.; Cerrizuela, Santiago; Tribulo, Celeste; Aybar, Manuel J. (May 2018). "Neurocristopathies: New insights 150 years after the neural crest discovery". Developmental Biology. 444: S110–S143. doi: 10.1016/j.ydbio.2018.05.013 . hdl: 11336/101713 . ISSN 0012-1606. PMID 29802835.
- ↑ Bolande, Robert P. (1974). "The neurocristopathies: A unifying concept of disease arising in neural crest maldevelopment". Human Pathology. 5 (4): 409–29. doi:10.1016/S0046-8177(74)80021-3.
- ↑ Watt, Kristin E. Noack; Trainor, Paul A. (2014), "Neurocristopathies", Neural Crest Cells, Elsevier, pp. 361–394, doi:10.1016/b978-0-12-401730-6.00018-1, ISBN 9780124017306
- ↑ Cerrizuela, Santiago; Vega-Lopez, Guillermo A.; Aybar, Manuel J. (2020). "The role of teratogens in neural crest development". Birth Defects Research. 112 (8): 584–632. doi:10.1002/bdr2.1644. ISSN 2472-1727. PMID 31926062. S2CID 210151171.
- ↑ Vega-Lopez, Guillermo A.; Cerrizuela, Santiago; Tribulo, Celeste; Aybar, Manuel J. (May 2018). "Neurocristopathies: New insights 150 years after the neural crest discovery". Developmental Biology. 444: S110–S143. doi: 10.1016/j.ydbio.2018.05.013 . hdl: 11336/101713 . ISSN 0012-1606. PMID 29802835.
- ↑ Behan, Peter O.; Chaudhuri, Abhijit (2010). "The sad plight of multiple sclerosis research (low on fact, high on fiction): Critical data to support it being a neurocristopathy". Inflammopharmacology. 18 (6): 265–90. doi:10.1007/s10787-010-0054-4. PMID 20862553. S2CID 11711382.