Related Research Articles
Alkaliphiles are a class of extremophilic microbes capable of survival in alkaline environments, growing optimally around a pH of 10. These bacteria can be further categorized as obligate alkaliphiles, facultative alkaliphiles and haloalkaliphiles.

Swarming motility is a rapid and coordinated translocation of a bacterial population across solid or semi-solid surfaces, and is an example of bacterial multicellularity and swarm behaviour. Swarming motility was first reported by Jorgen Henrichsen and has been mostly studied in genus Serratia, Salmonella, Aeromonas, Bacillus, Yersinia, Pseudomonas, Proteus, Vibrio and Escherichia.

Multidrug and toxin extrusion protein 2 is a protein which in humans is encoded by the SLC47A2 gene.
Roberto Kolter is Professor of Microbiology, Emeritus at Harvard Medical School, an author, and past president of the American Society for Microbiology. Kolter has been a professor at Harvard Medical School since 1983 and was Co-director of Harvard's Microbial Sciences Initiative from 2003-2018. During the 35-year term of the Kolter laboratory from 1983 to 2018, more than 130 graduate student and postdoctoral trainees explored an eclectic mix of topics gravitating around the study of microbes. Kolter is a fellow of the American Association for the Advancement of Science and of the American Academy of Microbiology.
The Nucleobase cation symporter-2 (NCS2) family, also called the Nucleobase ascorbate transporter (NAT) family, consists of over 1000 sequenced proteins derived from gram-negative and gram-positive bacteria, archaea, fungi, plants and animals. The NCS2/NAT family is a member of the APC Superfamily of secondary carriers. Of the five known families of transporters that act on nucleobases, NCS2/NAT is the only one that is most widespread. Many functionally characterized members are specific for nucleobases including both purines and pyrimidines, but others are purine-specific. However, two closely related rat/human members of the family, SVCT1 and SVCT2, localized to different tissues of the body, co-transport L-ascorbate (vitamin C) and Na+ with a high degree of specificity and high affinity for the vitamin. Clustering of NCS2/NAT family members on the phylogenetic tree is complex, with bacterial proteins and eukaryotic proteins each falling into at least three distinct clusters. The plant and animal proteins cluster loosely together, but the fungal proteins branch from one of the three bacterial clusters forming a tighter grouping. E. coli possesses four distantly related paralogous members of the NCS2 family.
Cation diffusion facilitators (CDFs) are transmembrane proteins that provide tolerance of cells to divalent metal ions, such as cadmium, zinc, and cobalt. These proteins are considered to be efflux pumps that remove these divalent metal ions from cells. However, some members of the CDF superfamily are implicated in ion uptake. All members of the CDF family possess six putative transmembrane spanners with strongest conservation in the four N-terminal spanners. The Cation Diffusion Facilitator (CDF) Superfamily includes the following families:
The bacterial murein precursor exporter (MPE) family is a member of the cation diffusion facilitator (CDF) superfamily of membrane transporters. Members of the MPE family are found in a large variety of Gram-negative and Gram-positive bacteria and facilitate the translocation of lipid-linked murein precursors. A representative list of proteins belonging to the MPE family can be found in the Transporter Classification Database.
Lysine Exporters are a superfamily of transmembrane proteins which export amino acids, lipids and heavy metal ions. They provide ionic homeostasis, play a role in cell envelope assembly, and protect from excessive concentrations of heavy metals in cytoplasm. The superfamily was named based on the early discovery of the LysE carrier protein of Corynebacterium glutamicum.
The gluconate:H+ symporter (GntP) family (TC# 2.A.8) is a family of transport proteins belonging to the ion transporter (IT) superfamily. Members of the GntP family include known gluconate permeases of E. coli and Bacillus species such as the D-Gluconate:H+ symporter of Bacillus subtillus (GntP; TC# 2.A.8.1.1) and the D-fructuronate/D-gluconate:H+ symporter of E. coli (GntP; TC# 2.A.8.1.3). A representative list of proteins belonging to the GntP family can be found in the Transporter Classification Database.
Divalent anion:Na+ symporters were found in bacteria, archaea, plant chloroplasts and animals.
The Citrate-Mg2+:H+ (CitM) / Citrate-Ca2+:H+ (CitH) Symporter (CitMHS) Family (TC# 2.A.11) is a family of transport proteins belonging to the Ion transporter superfamily. Members of this family are found in Gram-positive and Gram-negative bacteria, archaea and possibly eukaryotes. These proteins all probably arose by an internal gene duplication event. Lensbouer & Doyle (2010) have reviewed these systems, classifying the porters with three superfamilies, according to ion-preference:
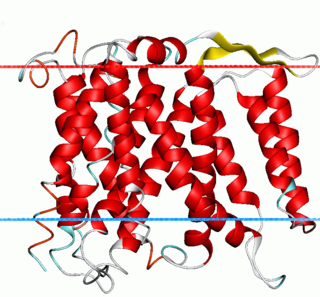
Na+/H+ antiporter A (NhaA) family (TC# 2.A.33) contains a number of bacterial sodium-proton antiporter (SPAP) proteins. These are integral membrane proteins that catalyse the exchange of H+ for Na+ in a manner that is highly pH dependent. Homologues have been sequenced from a number of bacteria and archaea. Prokaryotes possess multiple paralogues. A representative list of the proteins that belong to the NhaA family can be found in the Transporter Classification Database.
The NhaB family belongs to the ion transporter (IT) superfamily. A representative list of proteins belonging to the NhaB family can be found in the Transporter Classification Database.
The NhaD family belongs to the Ion Transporter (IT) Superfamily. A representative list of proteins belonging to the NhaD family can be found in the Transporter Classification Database.
The Monovalent Cation:Proton Antiporter-2 (CPA2) Family is a moderately large family of transporters belonging to the CPA superfamily. Members of the CPA2 family have been found in bacteria, archaea and eukaryotes. The proteins of the CPA2 family consist of between 333 and 900 amino acyl residues and exhibit 10-14 transmembrane α-helical spanners (TMSs).
The Monovalent Cation (K+ or Na+):Proton Antiporter-3 (CPA3) Family (TC# 2.A.63) is a member of the Na+ transporting Mrp superfamily. The CPA3 family consists of bacterial multicomponent K+:H+ and Na+:H+ antiporters. The best characterized systems are the PhaABCDEFG system of Sinorhizobium meliloti (TC# 2.A.63.1.1) that functions in pH adaptation and as a K+ efflux system, and the MnhABCDEFG system of Staphylococcus aureus (TC# 2.A.63.1.3) that functions as a Na+ efflux Na+:H+ antiporter.
The Bacillus subtilis φ29 Holin Family is a group of transporters belonging to the Holin Superfamily IV. A representative list of members belonging to the φ29 holin family can be found in the Transporter Classification Database.
The Bacillus Spore Morphogenesis and Germination Holin (BSH) Family is a family of proteins named after a holin in Bacillus subtilis described to be involved in spore morphogenesis and germination by Real et al (2005). The gene encoding this holin is ywcE. Mutants lacking this gene or its product have spores that exhibit outer coat defects. These spores lack the characteristic striatal pattern resulting in the failure of the outer coat to attach to the underlying inner coat. Finally, the mutant spores accumulate reduced amounts of dipicolinic acid. BSH proteins average about 90 amino acyl residues in length and exhibit 3 transmembrane segments (TMSs). A representative list of homologous proteins, found only in Bacillus species, is available in the Transporter Classification Database.

The Phosphate (Pho) regulon is a bacterial regulatory mechanism used for the conservation and management of inorganic phosphate within the cell. It was first discovered in Escherichia coli as an operating system for the bacterial strain, and was later identified in other species. The Pho system is composed of various components including extracellular enzymes and transporters that are capable of phosphate assimilation in addition to extracting inorganic phosphate from organic sources. This is an essential process since phosphate plays an important role in cellular membranes, genetic expression, and metabolism within the cell. Under low nutrient availability, the Pho regulon helps the cell survive and thrive despite a depletion of phosphate within the environment. When this occurs, phosphate starvation-inducible (psi) genes activate other proteins that aid in the transport of inorganic phosphate.
The Bacteroides thetaiotaomicron genome contains hundreds of small RNAs (sRNAs), discovered through RNA sequencing. These include canonical housekeeping RNA species such as the 6S RNA (SsrS), tmRNA (SsrA), M1 RNA (RnpB) and 4.5S RNA (Ffs) as well as several hundred cis and trans encoded small RNAs. More than 20 candidates have been validated with northern blots and the structures of several members have been characterized through in silico analyses and chemical probing experiments.
References
- ↑ Prakash, Shraddha; Cooper, Garret; Singhi, Soumya; Saier, Milton H. (2003-12-03). "The ion transporter superfamily". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1618 (1): 79–92. doi: 10.1016/j.bbamem.2003.10.010 . ISSN 0006-3002. PMID 14643936.
- ↑ Ito, M.; Guffanti, A. A.; Zemsky, J.; Ivey, D. M.; Krulwich, T. A. (1997-06-01). "Role of the nhaC-encoded Na+/H+ antiporter of alkaliphilic Bacillus firmus OF4". Journal of Bacteriology. 179 (12): 3851–3857. doi:10.1128/jb.179.12.3851-3857.1997. ISSN 0021-9193. PMC 179192 . PMID 9190799.
- ↑ Wei, Y.; Guffanti, A. A.; Ito, M.; Krulwich, T. A. (2000-09-29). "Bacillus subtilis YqkI is a novel malic/Na+-lactate antiporter that enhances growth on malate at low protonmotive force". The Journal of Biological Chemistry. 275 (39): 30287–30292. doi: 10.1074/jbc.M001112200 . ISSN 0021-9258. PMID 10903309.
As of this edit, this article uses content from "2.A.35 The NhaC Na+:H+ Antiporter (NhaC) Family" , which is licensed in a way that permits reuse under the Creative Commons Attribution-ShareAlike 3.0 Unported License, but not under the GFDL. All relevant terms must be followed.