
Mycobacterium is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis and leprosy in humans. The Greek prefix myco- means 'fungus', alluding to this genus' mold-like colony surfaces. Since this genus has cell walls with a waxy lipid-rich outer layer that contains high concentrations of mycolic acid, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types.
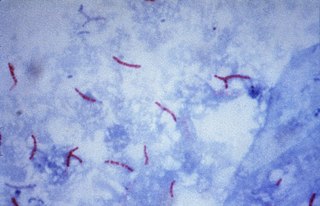
The Ziehl-Neelsen stain, also known as the acid-fast stain, is a bacteriological staining technique used in cytopathology and microbiology to identify acid-fast bacteria under microscopy, particularly members of the Mycobacterium genus. This staining method was initially introduced by Paul Ehrlich (1854–1915) and subsequently modified by the German bacteriologists Franz Ziehl (1859–1926) and Friedrich Neelsen (1854–1898) during the late 19th century.

Mycobacterium smegmatis is an acid-fast bacterial species in the phylum Actinomycetota and the genus Mycobacterium. It is 3.0 to 5.0 µm long with a bacillus shape and can be stained by Ziehl–Neelsen method and the auramine-rhodamine fluorescent method. It was first reported in November 1884 by Lustgarten, who found a bacillus with the staining appearance of tubercle bacilli in syphilitic chancres. Subsequent to this, Alvarez and Tavel found organisms similar to that described by Lustgarten also in normal genital secretions (smegma). This organism was later named M. smegmatis.
The oxidase test is used to determine whether an organism possesses the cytochrome c oxidase enzyme. The test is used as an aid for the differentiation of Neisseria, Moraxella, Campylobacter and Pasteurella species. It is also used to differentiate pseudomonads from related species.
Mycobacterium branderi is a slowly growing, nonchromogenic Mycobacterium first isolated from patients in Finland. Etymology: of Brander, referring to Eljas Brander, the former head of the Tuberculosis Laboratory of the National Public Health Institute, Finland, who collected the strains.

Mycobacterium cosmeticum is a rapidly growing mycobacterium that was first isolated from cosmetic patients and sites performing cosmetic procedures.
Mycobacterium elephantis, a bacterium of the family Mycobacteriaceae, was discovered and isolated from a deceased elephant near India and may be linked to respiratory dysfunction. Organisms in the genus Mycobacterium are known to be aerobic and non-motile. Organisms within Mycobacterium belong to either the rapid growing group or the slow growing group. M. elephantis is classified as a rapid grower and relates most closely to Mycobacterium confluentis and Mycobacterium phlei.
Mycobacterium fallax is a species of the phylum Actinomycetota, belonging to the genus Mycobacterium.

Mycobacterium fortuitum is a nontuberculous species of the phylum Actinomycetota, belonging to the genus Mycobacterium.
Mycobacterium gastri is a species of the phylum Actinomycetota, belonging to the genus Mycobacterium.
Mycobacterium gordonae is a species of Mycobacterium named for Ruth E. Gordon. It is a species of the phylum Actinomycetota, belonging to the genus Mycobacterium.
Mycobacterium hassiacum is a rapid-growing thermophilic mycobacterium that was isolated in human urine in 1997 by researchers at the German University of Regensburg. It's a species of the phylum Actinomycetota, belonging to the genus Mycobacterium.
Mycobacterium hodleri is a species of the phylum Actinomycetota, belonging to the genus Mycobacterium.

Mycobacterium kansasii is a bacterium in the Mycobacterium genus. It is an environmental bacteria that causes opportunistic infections in humans, and is one of the leading mycobacterial causes of human disease after tuberculosis and leprosy.
In microbiology, the phenotypic testing of mycobacteria uses a number of methods. The most-commonly used phenotypic tests to identify and distinguish Mycobacterium strains and species from each other are described below.

Löwenstein–Jensen medium, more commonly known as LJ medium, is a growth medium specially used for culture of Mycobacterium species, notably Mycobacterium tuberculosis.

The IMViC tests are a group of individual tests used in microbiology lab testing to identify an organism in the coliform group. A coliform is a gram negative, aerobic, or facultative anaerobic rod, which produces gas from lactose within 48 hours. The presence of some coliforms indicate fecal contamination.
Mycolicibacter terrae is a slow-growing species of mycobacteria. It is an ungrouped member of the third Runyon. It is known to cause serious skin infections, which are "relatively resistant to antibiotic therapy".

Mycobacteria Growth Indicator Tube (MGIT) is intended for the culture, detection and recovery of mycobacteria. The MGIT Mycobacteria Growth Indicator Tube contains 7 mL of modified Middlebrook 7H9 Broth base. The complete medium, with OADC enrichment and PANTA antibiotic mixture, is one of the most commonly used liquid media for the cultivation of mycobacteria.
Diagnostic microbiology is the study of microbial identification. Since the discovery of the germ theory of disease, scientists have been finding ways to harvest specific organisms. Using methods such as differential media or genome sequencing, physicians and scientists can observe novel functions in organisms for more effective and accurate diagnosis of organisms. Methods used in diagnostic microbiology are often used to take advantage of a particular difference in organisms and attain information about what species it can be identified as, which is often through a reference of previous studies. New studies provide information that others can reference so that scientists can attain a basic understanding of the organism they are examining.










