Related Research Articles
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
Memory T cells are a subset of T lymphocytes that might have some of the same functions as memory B cells. Their lineage is unclear.

Steven A. Rosenberg is an American cancer researcher and surgeon, chief of Surgery at the National Cancer Institute in Bethesda, Maryland and a Professor of Surgery at the Uniformed Services University of Health Sciences and the George Washington University School of Medicine and Health Sciences. He pioneered the development of immunotherapy that has resulted in the first effective immunotherapies and the development of gene therapy. He is the first researcher to successfully insert foreign genes into humans.

Tumor antigen is an antigenic substance produced in tumor cells, i.e., it triggers an immune response in the host. Tumor antigens are useful tumor markers in identifying tumor cells with diagnostic tests and are potential candidates for use in cancer therapy. The field of cancer immunology studies such topics.
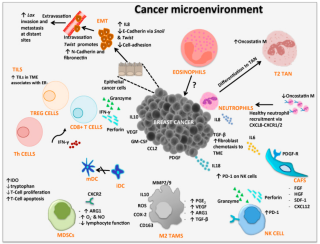
Cancer immunology is an interdisciplinary branch of biology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Tumor-infiltrating lymphocytes (TIL) are white blood cells that have left the bloodstream and migrated towards a tumor. They include T cells and B cells and are part of the larger category of ‘tumor-infiltrating immune cells’ which consist of both mononuclear and polymorphonuclear immune cells, in variable proportions. Their abundance varies with tumor type and stage and in some cases relates to disease prognosis.
Adoptive immunity acts in a host after their immunological components are withdrawn, their immunological activity is modified extracorporeally, and then reinfused into the same host. This process in its former part is analogous to adoption: a child is once adopted out from their home, grown up, and then returned to their home of birth. Transferred immunological components include immune cells such as T lymphocytes or tumour-infiltrating lymphocytes, NK cells, macrophages, or B cells.
Adoptive cell transfer (ACT) is the transfer of cells into a patient. The cells may have originated from the patient or from another individual. The cells are most commonly derived from the immune system with the goal of improving immune functionality and characteristics. In autologous cancer immunotherapy, T cells are extracted from the patient, genetically modified and cultured in vitro and returned to the same patient. Comparatively, allogeneic therapies involve cells isolated and expanded from a donor separate from the patient receiving the cells.
Gustav Gaudernack is a scientist working in the development of cancer vaccines and cancer immunotherapy. He has developed various strategies in immunological treatment of cancer. He is involved in several ongoing cellular and immuno-gene therapeutic clinical trials and his research group has put major efforts into the development of various T cell-based immunotherapeutic strategies.
Tumor-associated macrophages (TAMs) are a class of immune cells present in high numbers in the microenvironment of solid tumors. They are heavily involved in cancer-related inflammation. Macrophages are known to originate from bone marrow-derived blood monocytes or yolk sac progenitors, but the exact origin of TAMs in human tumors remains to be elucidated. The composition of monocyte-derived macrophages and tissue-resident macrophages in the tumor microenvironment depends on the tumor type, stage, size, and location, thus it has been proposed that TAM identity and heterogeneity is the outcome of interactions between tumor-derived, tissue-specific, and developmental signals.
Dmitry Gabrilovich is currently a Chief Scientist, Cancer Immunology at AstraZeneca in Gaithersburg, MD, USA. His research is focused on methods by which tumors are able to suppress the immune system and how to develop new immune therapies to combat this ability. Gabrilovich described the defective ability of dendritic cells in induce immune responses in cancer, and was one of the discoverers of myeloid-derived suppressor cells (MDSC). The Gabrilovich lab focuses on immature myeloid cell biology and its relation to cancer. MDSC have been linked to a number of signaling pathways associated with cancer, including NF-κB, Jak-STAT, Notch, Wnt, and Rb, among others. His research has found that tumor cells can go through a mechanism that produces a free radical peroxynitrite, causing them to become resistant to certain types of cancer immunotherapy. His research has also focused on monocytic-myeloid derived suppressor cells and polymorphonuclear-myeloid derived suppressor cells and what impact they might have on cancer therapy, since myeloid derived suppressor cells negatively regulate anti-tumor activity. His group described methods of targeting suppressive myeloid cell. In 2019 he was awarded title of Research Professor by American Cancer Society. Prior to joining AstraZeneca, Gabrilovich was a researcher and Christopher M. Davis professor at The Wistar Institute in Philadelphia. Prior to joining The Wistar Institute, Gabrilovich was a senior member at the Moffitt Cancer Center in Tampa.

Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.
Checkpoint inhibitor therapy is a form of cancer immunotherapy. The therapy targets immune checkpoints, key regulators of the immune system that when stimulated can dampen the immune response to an immunologic stimulus. Some cancers can protect themselves from attack by stimulating immune checkpoint targets. Checkpoint therapy can block inhibitory checkpoints, restoring immune system function. The first anti-cancer drug targeting an immune checkpoint was ipilimumab, a CTLA4 blocker approved in the United States in 2011.
The dendritic cell-based cancer vaccine is an innovation in therapeutic strategy for cancer patients.
The mutanome is the entirety of somatic cancer mutations in an individual tumor.
Neoepitopes are a class of major histocompatibility complex (MHC) bounded peptides. They represent the antigenic determinants of neoantigens. Neoepitopes are recognized by the immune system as targets for T cells and can elicit immune response to cancer.
Pamela Sumiko Ohashi, PhD, FRSC is a Canadian medical researcher. She is co-director of the Campbell Family Institute for Breast Cancer Research, director of the Cancer Immune Therapy Program at the Princess Margaret Cancer Centre and a professor at the University of Toronto.

George Coukos is a physician-scientist in tumor immunology, professor and director of the Ludwig Cancer Research Lausanne Branch and director of the Department of oncology UNIL-CHUV of the University of Lausanne and the Lausanne University Hospital in Lausanne, Switzerland. He is known for his work on the mechanisms by which tumors suppress anti-cancer immune responses, and the role of the tumor vasculature in that suppression. In addition to his work in ovarian cancer, the combinatorial immune therapies proposed by Professor Coukos have been successfully tested and approved for lung, liver and kidney cancers.
References
- ↑ "Nicholas P. Restifo, M.D." National Cancer Institute. September 12, 2009. Archived from the original on March 24, 2010. Retrieved April 14, 2010.
- ↑ "Nicholas P. Restifo, M.D. | Center for Cancer Research". ccr.cancer.gov. Archived from the original on 2015-09-12.
- ↑ Search Results for author Restifo NP on PubMed .
- ↑ "Nicholas P Restifo, MD".
- ↑ Rosenberg, Steven A.; Restifo, Nicholas P. (2015). "Adoptive cell transfer as personalized immunotherapy for human cancer". Science. 348 (6230): 62–68. Bibcode:2015Sci...348...62R. doi:10.1126/science.aaa4967. PMC 6295668 . PMID 25838374.
- ↑ Gattinoni, L., Lugli, E., Ji, Y. et al. A human memory T cell subset with stem cell–like properties. Nat Med 17, 1290–1297 (2011). https://doi.org/10.1038/nm.2446
- ↑ Nicholas P. Restifo, Francesco M. Marincola, Yutaka Kawakami, Jeff Taubenberger, John R. Yannelli, Steven A. Rosenberg, Loss of Functional Beta 2 -Microglobulin in Metastatic Melanomas From Five Patients Receiving Immunotherapy , JNCI: Journal of the National Cancer Institute, Volume 88, Issue 2, 17 January 1996, Pages 100–108, https://doi.org/10.1093/jnci/88.2.100
- ↑ Identification of Human Cancers Deficient in Antigen Processing
- ↑ DOI:https://doi.org/10.1016/j.it.2004.12.003
- ↑ Clever, D, et al. (Aug 25, 2016). "Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche". Cell. 166 (5): 1117–1131. doi:10.1016/j.cell.2016.07.032. PMC 5548538 . PMID 27565342.
- ↑ Eil, R, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016 Sep 14. doi: 10.1038/nature19364. [Epub ahead of print]
- 1 2 Restifo NP, Dudley ME, Rosenberg SA (March 2012). "Adoptive immunotherapy for cancer: harnessing the T cell response". Nature Reviews Immunology. 12 (4): 269–81. doi:10.1038/nri3191. PMC 6292222 . PMID 22437939.
- ↑ Restifo NP, Esquivel F, Kawakami Y, et al. (February 1993). "Identification of human cancers deficient in antigen processing". The Journal of Experimental Medicine. 177 (2): 265–72. doi:10.1084/jem.177.2.265. PMC 1950463 . PMID 8426105.
- ↑ Khong HT, Restifo NP (November 2002). "Natural selection of tumor variants in the generation of "tumor escape" phenotypes". Nature Immunology. 3 (11): 999–1005. doi:10.1038/ni1102-999. PMC 1508168 . PMID 12407407.
- ↑ Irvine KR, McCabe BJ, Rosenberg SA, Restifo NP (May 1995). "Synthetic Oligonucleotide Expressed by a Recombinant Vaccinia Virus Elicits Therapeutic CTL". Journal of Immunology. 154 (9): 4651–7. doi:10.4049/jimmunol.154.9.4651. PMC 1976247 . PMID 7722317.
- ↑ Gattinoni, Luca; Zhong, Xiao-Song; Palmer, Douglas C.; Ji, Yun; Hinrichs, Christian S.; Yu, Zhiya; Wrzesinski, Claudia; Boni, Andrea; Cassard, Lydie; Garvin, Lindsay M.; Paulos, Chrystal M.; Muranski, Pawel; Restifo, Nicholas P. (July 29, 2009). "Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells". Nature Medicine. 15 (7): 808–813. doi:10.1038/nm.1982. PMC 2707501 . PMID 19525962.