Related Research Articles

Ammonia is an inorganic compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.

Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.

Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, liquids, and organisms that together support life of plants and soil organisms. Some scientific definitions distinguish dirt from soil by restricting the former term specifically to displaced soil.

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.
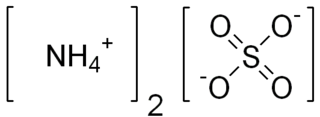
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid.
Edaphology is concerned with the influence of soils on living beings, particularly plants. It is one of two main divisions of soil science, the other being pedology. Edaphology includes the study of how soil influences humankind's use of land for plant growth as well as people's overall use of the land. General subfields within edaphology are agricultural soil science and environmental soil science.

Organic fertilizers are fertilizers that are naturally produced. Fertilizers are materials that can be added to soil or plants, in order to provide nutrients and sustain growth. Typical organic fertilizers include all animal waste including meat processing waste, manure, slurry, and guano; plus plant based fertilizers such as compost; and biosolids. Inorganic "organic fertilizers" include minerals and ash. The organic-mess refers to the Principles of Organic Agriculture, which determines whether a fertilizer can be used for commercial organic agriculture, not whether the fertilizer consists of organic compounds.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
Soil acidification is the buildup of hydrogen cations, which reduces the soil pH. Chemically, this happens when a proton donor gets added to the soil. The donor can be an acid, such as nitric acid, sulfuric acid, or carbonic acid. It can also be a compound such as aluminium sulfate, which reacts in the soil to release protons. Acidification also occurs when base cations such as calcium, magnesium, potassium and sodium are leached from the soil.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
The Kjeldahl method or Kjeldahl digestion (Danish pronunciation: [ˈkʰelˌtɛˀl]) in analytical chemistry is a method for the quantitative determination of nitrogen contained in organic substances plus the nitrogen contained in the inorganic compounds ammonia and ammonium (NH3/NH4+). Without modification, other forms of inorganic nitrogen, for instance nitrate, are not included in this measurement. Using an empirical relation between Kjeldahl nitrogen content and protein content it is an important method for analyzing proteins. This method was developed by Johan Kjeldahl in 1883.
Acid sulfate soils are naturally occurring soils, sediments or organic substrates that are formed under waterlogged conditions. These soils contain iron sulfide minerals and/or their oxidation products. In an undisturbed state below the water table, acid sulfate soils are benign. However, if the soils are drained, excavated or otherwise exposed to air, the sulfides react with oxygen to form sulfuric acid.

The history of fertilizer has largely shaped political, economic, and social circumstances in their traditional uses. Subsequently, there has been a radical reshaping of environmental conditions following the development of chemically synthesized fertilizers.

A fluvisol in the World Reference Base for Soil Resources (WRB) is a genetically young soil in alluvial deposits. Apart from river sediments, they also occur in lacustrine and marine deposits. Fluvisols correlate with fluvents and fluvaquents of the USDA soil taxonomy. The good natural fertility of most fluvisols and their attractive dwelling sites on river levees and higher parts in marine landscapes were recognized in prehistoric times.
Johannes "Johan" Bouma is a Dutch soil scientist. He worked at the Netherlands Soil Survey Institute from 1975 to 1983 and was professor of soil science at Wageningen University and Research Centre between 1983 and 2002.
References
- 1 2 "Genesis and solution chemistry of acid sulfate soils in Thailand" (PDF). Wageningen University and Research Centre. Archived from the original (PDF) on 15 January 2018.
- 1 2 3 "Van wetenschapper naar galeriehouder" (in Dutch). Resource WUR.
- 1 2 "Nieuwsbrief XI 2004" (PDF) (in Dutch). Nederlandse Bodemkundige Vereniging. Spring 2004. Archived from the original (PDF) on 29 December 2016.
- ↑ "Vieze stikstof". NRC Handelsblad (in Dutch). 24 February 2007. Archived from the original on 17 January 2018.
- ↑ "Mestbeleid succes, maar toch nog slag nodig" (in Dutch). Boerderij.nl. 11 August 2011. Archived from the original on 16 June 2016.
- ↑ Hans van Grinsven. "Bodemverzuring (1982)" (in Dutch). Nederlandse Bodemkundige Vereniging. Archived from the original on 22 October 2016.
- ↑ Sarah Graham (20 August 2002). "Rice Paddy Methane Emissions Depend on Crops' Success". Scientific American . Archived from the original on 19 May 2014.
- ↑ "Jaarverslag 2011 – 2012" (PDF) (in Dutch). Natuurwetenschappelijk Gezelschap Wageningen. September 2012. Archived from the original (PDF) on 22 April 2017.
- ↑ "Nico van Breemen". Royal Netherlands Academy of Arts and Sciences. Archived from the original on 15 January 2018.