Related Research Articles

Life extension is the concept of extending the human lifespan, either modestly through improvements in medicine or dramatically by increasing the maximum lifespan beyond its generally-settled biological limit of around 125 years. Several researchers in the area, along with "life extensionists", "immortalists", or "longevists", postulate that future breakthroughs in tissue rejuvenation, stem cells, regenerative medicine, molecular repair, gene therapy, pharmaceuticals, and organ replacement will eventually enable humans to have indefinite lifespans through complete rejuvenation to a healthy youthful condition (agerasia). The ethical ramifications, if life extension becomes a possibility, are debated by bioethicists.

Longevity may refer to especially long-lived members of a population, whereas life expectancy is defined statistically as the average number of years remaining at a given age. For example, a population's life expectancy at birth is the same as the average age at death for all people born in the same year.

Haptoglobin is the protein that in humans is encoded by the HP gene. In blood plasma, haptoglobin binds with high affinity to free hemoglobin released from erythrocytes, and thereby inhibits its deleterious oxidative activity. Compared to Hp, hemopexin binds to free heme. The haptoglobin-hemoglobin complex will then be removed by the reticuloendothelial system.
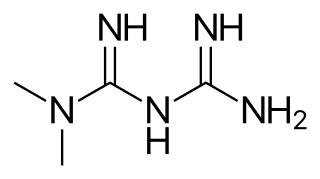
Metformin, sold under the brand name Glucophage, among others, is the main first-line medication for the treatment of Type 2 diabetes, particularly in people who are overweight. It is also used in the treatment of polycystic ovary syndrome, and is sometimes used as an off-label adjunct to lessen the risk of metabolic syndrome in people who take antipsychotics. It has also been shown to inhibit inflammation, and is not associated with weight gain. Metformin is taken orally.
Heteroplasmy is the presence of more than one type of organellar genome within a cell or individual. It is an important factor in considering the severity of mitochondrial diseases. Because most eukaryotic cells contain many hundreds of mitochondria with hundreds of copies of mitochondrial DNA, it is common for mutations to affect only some mitochondria, leaving most unaffected.
Cholesteryl ester transfer protein (CETP), also called plasma lipid transfer protein, is a plasma protein that facilitates the transport of cholesteryl esters and triglycerides between the lipoproteins. It collects triglycerides from very-low-density (VLDL) or Chylomicrons and exchanges them for cholesteryl esters from high-density lipoproteins (HDL), and vice versa. Most of the time, however, CETP does a heteroexchange, trading a triglyceride for a cholesteryl ester or a cholesteryl ester for a triglyceride.

Apolipoprotein E (Apo-E) is a protein involved in the metabolism of fats in the body of mammals. A subtype is implicated in Alzheimer's disease and cardiovascular diseases. It is encoded in humans by the gene APOE.

Battenin is a protein that in humans is encoded by the CLN3 gene located on chromosome 16. Battenin is not clustered into any Pfam clan, but it is included in the TCDB suggesting that it is a transporter. In humans, it belongs to the atypical SLCs due to its structural and phylogenetic similarity to other SLC transporters.

In genomics, a genome-wide association study, is an observational study of a genome-wide set of genetic variants in different individuals to see if any variant is associated with a trait. GWA studies typically focus on associations between single-nucleotide polymorphisms (SNPs) and traits like major human diseases, but can equally be applied to any other genetic variants and any other organisms.

Forkhead box O3, also known as FOXO3 or FOXO3a, is a human protein encoded by the FOXO3 gene.

Sirtuin 1, also known as NAD-dependent deacetylase sirtuin-1, is a protein that in humans is encoded by the SIRT1 gene.
Paola Sebastiani is a biostatistician and a professor at Boston University working in the field of genetic epidemiology, building prognostic models that can be used for the dissection of complex traits. Her research interests include Bayesian modeling of biomedical data, particularly genetic and genomic data.
A centenarian is a person who has attained the age of 100 years or more. Research on centenarians has become more common with clinical and general population studies now having been conducted in France, Hungary, Japan, Italy, Finland, Denmark, the United States, and China. Centenarians are the second fastest-growing demographic in much of the developed world. By 2030, it is expected that there will be around a million centenarians worldwide. In the United States, a 2010 Census Bureau report found that more than 80 percent of centenarians are women.
The medical genetics of Jews have been studied to identify and prevent some rare genetic diseases that, while still rare, are more common than average among people of Jewish descent. There are several autosomal recessive genetic disorders that are more common than average in ethnically Jewish populations, particularly Ashkenazi Jews, because of relatively recent population bottlenecks and because of consanguineous marriage. These two phenomena reduce genetic diversity and raise the chance that two parents will carry a mutation in the same gene and pass on both mutations to a child.
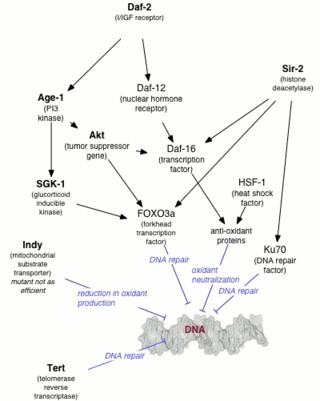
Genetics of aging is generally concerned with life extension associated with genetic alterations, rather than with accelerated aging diseases leading to reduction in lifespan.

Humanin is a micropeptide encoded in the mitochondrial genome by the 16S ribosomal RNA gene, MT-RNR2. Its structure contains a three-turn α-helix, and no symmetry.
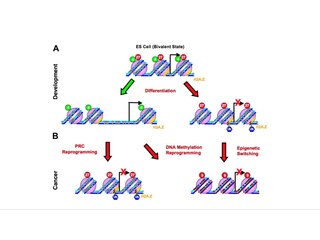
Epigenetic therapy refers to the use of drugs or other interventions to modify gene expression patterns, potentially treating diseases by targeting epigenetic mechanisms such as DNA methylation and histone modifications.
In biogerontology, the disposable soma theory of aging states that organisms age due to an evolutionary trade-off between growth, reproduction, and DNA repair maintenance. Formulated by British biologist Thomas Kirkwood, the disposable soma theory explains that an organism only has a limited amount of resources that it can allocate to its various cellular processes. Therefore, a greater investment in growth and reproduction would result in reduced investment in DNA repair maintenance, leading to increased cellular damage, shortened telomeres, accumulation of mutations, compromised stem cells, and ultimately, senescence. Although many models, both animal and human, have appeared to support this theory, parts of it are still controversial. Specifically, while the evolutionary trade-off between growth and aging has been well established, the relationship between reproduction and aging is still without scientific consensus, and the cellular mechanisms largely undiscovered.

Katalin Susztak (Suszták) is a Hungarian American scientist and nephrologist at the Perelman School of Medicine at the University of Pennsylvania. She is a professor of medicine and genetics, and currently the codirector of the Complications Unit at the Institute for Diabetes, Obesity and Metabolism. Her laboratory made major contributions to the current understanding of kidney disease development. She is also the founder of the Transformative Research In DiabEtic NephropaThy (TRIDENT), a collaborative network of physicians and basic scientists, to find cures for diabetic kidney disease.
Itsik Pe'er is a computational biologist and a Full Professor in the Department of Computer Science at Columbia University.
References
- ↑ "Institute for Aging Research". Albert Einstein College of Medicine. Archived from the original on October 29, 2010. Retrieved April 29, 2014.
- ↑ Dreifus, Claudia (February 24, 2004). "A CONVERSATION WITH/Nir Barzilai; It's Not the Yogurt: Looking for Longevity Genes". New York Times. New York, NY. Retrieved April 29, 2014.
- ↑ deGrasse Tyson, Neil (host); Cohen, Chad (correspondent) (Jan 2007). "Aging". NOVA scienceNOW. PBS.
{{cite episode}}:|first2=has generic name (help) - ↑ Green, Jesse (November 6, 2011). "What Do a Bunch of Old Jews Know About Living Forever?". New York (magazine). NY, NY. Retrieved April 30, 2013.
- ↑ Barzilai, N; Atzmon, G; Schechter, C; Schaefer, EJ; Cupples, AL; Lipton, R; Cheng, S; Shuldiner, AR (October 15, 2003). "Unique lipoprotein phenotype and genotype associated with exceptional longevity". JAMA. 290 (15): 2030–2040. doi:10.1001/jama.290.15.2030. PMID 14559957.
- ↑ Atzmon, G; Rincon, M; Schechter, C; Shuldiner, An; Lipton, R; Bergman, A; Barzilai, N (April 4, 2006). "Lipoprotein Genotype and Conserved Pathway for Exceptional Longevity in Humans". PLOS Biol. 4 (4): e113. doi: 10.1371/journal.pbio.0040113 . PMC 1413567 . PMID 16602826.

- ↑ Atzmon, G; Polin, TI; Crandall, J; Tanner, K; Schechter, CB; Scherer, PE; Rincon, M; Siegel, G; Katz, M; Lipton, RB; Shuldiner, AR; Barzilai, N (2008). "Adiponectin levels and genotype: A potential regulator of life-span in humans". Journal of Gerontology: Biological Sciences. 63 (5): 447–453. doi:10.1093/gerona/63.5.447. PMC 4507412 . PMID 18511746.
- ↑ Atzmon, G; Barzilai, N; Hollowell, JG; Surks, MI; Gabriely, I (October 16, 2009). "Genetic Predisposition to Elevated Serum Thyrotropin is Associated with Exceptional Longevity". The Journal of Clinical Endocrinology & Metabolism. 94 (12): 4768–4775. doi:10.1210/jc.2009-0808. PMC 2795660 . PMID 19837933.
- ↑ Suh, Y; Atzmon, G; Cho, M-O; Hwang, D; Liu, B; Leahy, D; Barzilai, N; Cohen, P (March 4, 2008). "Functionally-significant insulin-like growth factor-I receptor mutations in centenarian". PNAS. 105 (9): 3438–3442. Bibcode:2008PNAS..105.3438S. doi: 10.1073/pnas.0705467105 . PMC 2265137 . PMID 18316725.
- ↑ Carey, Benedict (May 21, 2009). "At the Bridge Table, Clues to a Lucid Old Age". The New York Times. Retrieved April 30, 2014.
- ↑ P. Cohen Eureka Grant (2013-03-06). "CohBar, Inc: Innovations in the Treatment of Age-Related Diseases". Cohbar.com. Retrieved 2014-04-24.
- ↑ Barzilai, N; Huffman, DM; Muzumdar, RH; Bartke, A (June 2012). "The critical role of metabolic pathways in aging". Diabetes. 61 (6): 1315–1322. doi:10.2337/db11-1300. PMC 3357299 . PMID 22618766 . Retrieved April 30, 2014.
- ↑ Barzilai, N; Guarente, L; Kirkwood, TB; Patridge, L; Rando, TA; Slagboom, PE (July 10, 2012). "The place of genetics in ageing research". Nature Reviews Genetics. 13 (8): 589–594. doi:10.1038/nrg3290. PMID 22777128. S2CID 23717998.
- ↑ Barzilai, Nir; Crandall, Jill P.; Kritchevsky, Stephen B.; Espeland, Mark A. (2016-06-14). "Metformin as a Tool to Target Aging". Cell Metabolism. 23 (6): 1060–1065. doi:10.1016/j.cmet.2016.05.011. ISSN 1550-4131. PMC 5943638 . PMID 27304507.
- ↑ Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, et al. (2018). "Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults". Aging Cell. 17 (2): e12723. doi:10.1111/acel.12723. PMC 5847877 . PMID 29383869.
- ↑ Kato, K; Zweig, R; Schechter, C; Verghese, J; Barzilai, N; Atzmon, G (March 23, 2013). "Personality, self-rated health, and cognition in centenarians: Do personality and self-rated health relate to cognitive function in advanced age?". Aging. 5 (3): 183–191. doi:10.18632/aging.100545. PMC 3629290 . PMID 23524310.
- ↑ "Dr. Nir Barzilai on the TAME Study: Targeting Aging with Metformin". Aging Research. June 30, 2020. Retrieved October 24, 2024.
- ↑ "Nir Barzilai CV" (PDF). Albert Einstein College of Medicine. Mar 31, 2012. Retrieved April 30, 2014.
- ↑ USA: Nir Barzilai (Technion, Hadassah), the man who wants to beat back aging