Related Research Articles

Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light is in the visible region, which gives the fluorescent substance a distinct color that can be seen only when exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after.

Microscopy is the technical field of using microscopes to view objects and areas of objects that cannot be seen with the naked eye. There are three well-known branches of microscopy: optical, electron, and scanning probe microscopy, along with the emerging field of X-ray microscopy.

A microscope is an instrument used to see objects that are too small to be seen by the naked eye. Microscopy is the science of investigating small objects and structures using such an instrument. Microscopic means invisible to the eye unless aided by a microscope.

In molecular biology and biotechnology, a fluorescent tag, also known as a fluorescent label or fluorescent probe, is a molecule that is attached chemically to aid in the detection of a biomolecule such as a protein, antibody, or amino acid. Generally, fluorescent tagging, or labeling, uses a reactive derivative of a fluorescent molecule known as a fluorophore. The fluorophore selectively binds to a specific region or functional group on the target molecule and can be attached chemically or biologically. Various labeling techniques such as enzymatic labeling, protein labeling, and genetic labeling are widely utilized. Ethidium bromide, fluorescein and green fluorescent protein are common tags. The most commonly labelled molecules are antibodies, proteins, amino acids and peptides which are then used as specific probes for detection of a particular target.

Fluorescence spectroscopy is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores.

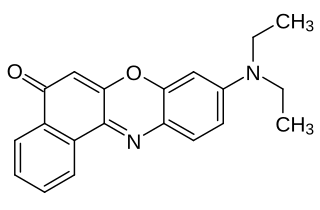
A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
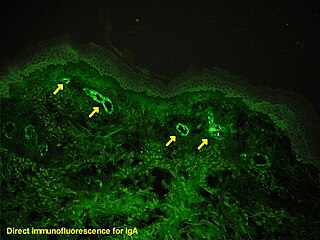
Immunofluorescence is a technique used for light microscopy with a fluorescence microscope and is used primarily on microbiological samples. This technique uses the specificity of antibodies to their antigen to target fluorescent dyes to specific biomolecule targets within a cell, and therefore allows visualization of the distribution of the target molecule through the sample. The specific region an antibody recognizes on an antigen is called an epitope. There have been efforts in epitope mapping since many antibodies can bind the same epitope and levels of binding between antibodies that recognize the same epitope can vary. Additionally, the binding of the fluorophore to the antibody itself cannot interfere with the immunological specificity of the antibody or the binding capacity of its antigen. Immunofluorescence is a widely used example of immunostaining and is a specific example of immunohistochemistry. This technique primarily makes use of fluorophores to visualise the location of the antibodies.
Plate readers, also known as microplate readers or microplate photometers, are instruments which are used to detect biological, chemical or physical events of samples in microtiter plates. They are widely used in research, drug discovery, bioassay validation, quality control and manufacturing processes in the pharmaceutical and biotechnological industry and academic organizations. Sample reactions can be assayed in 1-1536 well format microtiter plates. The most common microplate format used in academic research laboratories or clinical diagnostic laboratories is 96-well with a typical reaction volume between 100 and 200 µL per well. Higher density microplates are typically used for screening applications, when throughput and assay cost per sample become critical parameters, with a typical assay volume between 5 and 50 µL per well. Common detection modes for microplate assays are absorbance, fluorescence intensity, luminescence, time-resolved fluorescence, and fluorescence polarization.

A fluorescence microscope is an optical microscope that uses fluorescence and phosphorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a more simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image.
Nile red is a lipophilic stain. Nile red stains intracellular lipid droplets yellow. In most polar solvents, Nile red will not fluoresce; however, when in a lipid-rich environment can be intensely fluorescent, with varying colours from deep red to strong yellow-gold emission. The dye is highly solvatochromic and its emission and excitation wavelength both shift depending on solvent polarity and in polar media will hardly fluoresce at all.
A protein microarray is a high-throughput method used to track the interactions and activities of proteins, and to determine their function, and determining function on a large scale. Its main advantage lies in the fact that large numbers of proteins can be tracked in parallel. The chip consists of a support surface such as a glass slide, nitrocellulose membrane, bead, or microtitre plate, to which an array of capture proteins is bound. Probe molecules, typically labeled with a fluorescent dye, are added to the array. Any reaction between the probe and the immobilised protein emits a fluorescent signal that is read by a laser scanner. Protein microarrays are rapid, automated, economical, and highly sensitive, consuming small quantities of samples and reagents. The concept and methodology of protein microarrays was first introduced and illustrated in antibody microarrays in 1983 in a scientific publication and a series of patents. The high-throughput technology behind the protein microarray was relatively easy to develop since it is based on the technology developed for DNA microarrays, which have become the most widely used microarrays.

Fluorescence in situ hybridization (FISH) is a molecular cytogenetic technique that uses fluorescent probes that bind to only those parts of a nucleic acid sequence with a high degree of sequence complementarity. It was developed by biomedical researchers in the early 1980s to detect and localize the presence or absence of specific DNA sequences on chromosomes. Fluorescence microscopy can be used to find out where the fluorescent probe is bound to the chromosomes. FISH is often used for finding specific features in DNA for use in genetic counseling, medicine, and species identification. FISH can also be used to detect and localize specific RNA targets in cells, circulating tumor cells, and tissue samples. In this context, it can help define the spatial-temporal patterns of gene expression within cells and tissues.
Cyanine is the non-systematic name of a synthetic dye family belonging to polymethine group. The word cyanin is from the English word "cyan", which conventionally means a shade of blue-green and is derived from the Greek κυάνεος/κυανοῦς kyaneos/kyanous which means a somewhat different color: "dark blue".

Grace Bio-Labs is a global supplier of pharmaceutical, biomedical, and biochemical research products based in Bend, Oregon, United States. They develop the thin-cast nitrocellulose biochip and the modern hybridization and incubation chambers for glass microscope slides.
A reverse phase protein lysate microarray (RPMA) is a protein microarray designed as a dot-blot platform that allows measurement of protein expression levels in a large number of biological samples simultaneously in a quantitative manner when high-quality antibodies are available.
Fluorescence is used in the life sciences generally as a non-destructive way of tracking or analysing biological molecules by means of fluorescence. Some proteins or small molecules in cells are naturally fluorescent, which is called intrinsic fluorescence or autofluorescence. Alternatively, specific or general proteins, nucleic acids, lipids or small molecules can be "labelled" with an extrinsic fluorophore, a fluorescent dye which can be a small molecule, protein or quantum dot. Several techniques exist to exploit additional properties of fluorophores, such as fluorescence resonance energy transfer, where the energy is passed non-radiatively to a particular neighbouring dye, allowing proximity or protein activation to be detected; another is the change in properties, such as intensity, of certain dyes depending on their environment allowing their use in structural studies.
Photo-activated localization microscopy and stochastic optical reconstruction microscopy (STORM) are widefield fluorescence microscopy imaging methods that allow obtaining images with a resolution beyond the diffraction limit. The methods were proposed in 2006 in the wake of a general emergence of optical super-resolution microscopy methods, and were featured as Methods of the Year for 2008 by the Nature Methods journal. The development of PALM as a targeted biophysical imaging method was largely prompted by the discovery of new species and the engineering of mutants of fluorescent proteins displaying a controllable photochromism, such as photo-activatible GFP. However, the concomitant development of STORM, sharing the same fundamental principle, originally made use of paired cyanine dyes. One molecule of the pair, when excited near its absorption maximum, serves to reactivate the other molecule to the fluorescent state.
Time-resolved fluorescence energy transfer (TR-FRET) is the practical combination of time-resolved fluorometry (TRF) with Förster resonance energy transfer (FRET) that offers a powerful tool for drug discovery researchers. TR-FRET combines the low background aspect of TRF with the homogeneous assay format of FRET. The resulting assay provides an increase in flexibility, reliability and sensitivity in addition to higher throughput and fewer false positive/false negative results. FRET involves two fluorophores, a donor and an acceptor. Excitation of the donor by an energy source produces an energy transfer to the acceptor if the two are within a given proximity to each other. The acceptor in turn emits light at its characteristic wavelength.
Lanthanide probes are a non-invasive analytical tool commonly used for biological and chemical applications. Lanthanides are metal ions which have their 4f energy level filled and generally refer to elements cerium to lutetium in the periodic table. The fluorescence of lanthanide salts is weak because the energy absorption of the metallic ion is low; hence chelated complexes of lanthanides are most commonly used. The term chelate derives from the Greek word for “claw,” and is applied to name ligands, which attach to a metal ion with two or more donor atoms through dative bonds. The fluorescence is most intense when the metal ion has the oxidation state of 3+. Not all lanthanide metals can be used and the most common are: Sm(III), Eu(III), Tb(III), and Dy(III).
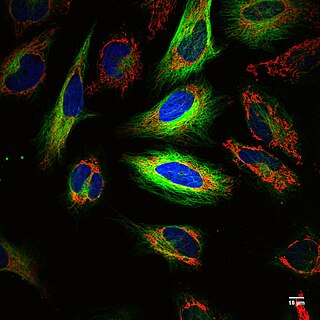
Fluorescence imaging is a type of non-invasive imaging technique that can help visualize biological processes taking place in a living organism. Images can be produced from a variety of methods including: microscopy, imaging probes, and spectroscopy.
References
- ↑ McGrath CM, Grudzien JL, Decker DA, Robbins TO (September 1991). "Cytometrically coherent transfer of receptor proteins on microporous membranes". BioTechniques. 11 (3): 352–4, 356, 358–61. PMID 1718329.
- ↑ McGrath CM, Grudzien JL, Levine AJ (1995). "High-Definition Cell Analysis In Situ Using Microporous Films". Cell Vision. 11 (3): 499–509. ISSN 1073-1180.
- ↑ Sauer, Ursula (2011). "Impact of Substrates for Probe Immobilization". 785: 363–378. doi:10.1007/978-1-61779-286-1_24. ISSN 1064-3745.Cite journal requires
|journal=(help) - ↑ Hollas, M; Jallerat, E; Pflanz, K; Praulich, I; Walter, J; Stahl, F; Scheper, T (2006). "New 3D black substrate for protein microarrays with improved dynamic range". Desalination. 199: 230. doi:10.1016/j.desal.2006.03.055.
- ↑ "SCHOTT Partners with Sartorius Stedim Biotech to Develop New Range of Nitrocellulose Coated Slides for Protein Microarrays" (PDF). The Nexterion Newsletter. 3: 4–5. September 2007.[ self-published source? ]
- ↑ Marie‐Laure Schneider, Adriana Marquez‐ Lagraulet, Richard Pasquesi, Michael Shultz - (2014), "Infrared detection decreases nitrocellulose auto-fluorescence and improves RPPA assays signal‐to‐noise ratio over visible wavelength detection", Innopsys Inc. Chicago, Illinois,[ self-published source? ]