
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.

In chemistry, an acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their application in solving related problems; these are called the acid–base theories, for example, Brønsted–Lowry acid–base theory.
The Ostwald process is a chemical process used for making nitric acid (HNO3). Wilhelm Ostwald developed the process, and he patented it in 1902. The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw material for the most common type of fertilizer production. Historically and practically, the Ostwald process is closely associated with the Haber process, which provides the requisite raw material, ammonia (NH3).

In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, a.k.a. phosphoric acid H3PO4.

Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula KNO
3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or nitre in the UK). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpeter (or saltpetre in the UK).

Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

Phosphoric acid is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula H3PO4. It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non-volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers.

Nitrification is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. The process of complete nitrification may occur through separate organisms or entirely within one organism, as in comammox bacteria. The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea.
An oxyanion, or oxoanion, is an ion with the generic formula A
xOz−
y. Oxyanions are formed by a large majority of the chemical elements. The formulae of simple oxyanions are determined by the octet rule. The corresponding oxyacid of an oxyanion is the compound H
zA
xO
y. The structures of condensed oxyanions can be rationalized in terms of AOn polyhedral units with sharing of corners or edges between polyhedra. The oxyanions adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are important in biology.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.
The sodium fusion test, or Lassaigne's test, is used in elemental analysis for the qualitative determination of the presence of foreign elements, namely halogens, nitrogen, and sulfur, in an organic compound. It was developed by J. L. Lassaigne.

Calcium nitrate, also called Norgessalpeter or Norwegian salpeter, is an inorganic compound with the formula Ca(NO3)2(H2O)x. The anhydrous compound, which is rarely encountered, absorbs moisture from the air to give the tetrahydrate. Both anhydrous and hydrated forms are colourless salts. Calcium nitrate is mainly used as a component in fertilizers, but it has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of double salts are known including calcium ammonium nitrate decahydrate (NH4NO3·5Ca(NO3)2·10H2O) and calcium potassium nitrate (Ca(NO3)2·4KNO3).
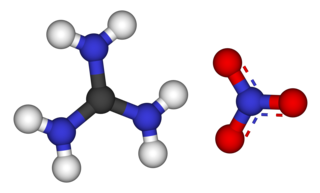
Guanidine nitrate is the chemical compound with the formula [C(NH2)3]NO3. It is a colorless, water-soluble salt. It is produced on a large scale and finds use as precursor for nitroguanidine, fuel in pyrotechnics and gas generators. Its correct name is guanidinium nitrate, but the colloquial term guanidine nitrate is widely used.

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

In chemistry, a phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO4 tetrahedra may be connected by shared single-bonded oxygens, forming linear or branched chains, cycles, or more complex structures. The single-bonded oxygen atoms that are not shared are completed with acidic hydrogen atoms. The general formula of a phosphoric acid is Hn+2−2xPnO3n+1−x, where n is the number of phosphorus atoms and x is the number of fundamental cycles in the molecule's structure, between 0 and n + 2/2.

Monocalcium phosphate is an inorganic compound with the chemical formula Ca(H2PO4)2 ("AMCP" or "CMP-A" for anhydrous monocalcium phosphate). It is commonly found as the monohydrate ("MCP" or "MCP-M"), Ca(H2PO4)2·H2O. Both salts are colourless solids. They are used mainly as superphosphate fertilizers and are also popular leavening agents.

Nitratine or nitratite, also known as cubic niter (UK: nitre), soda niter or Chile saltpeter (UK: Chile saltpetre), is a mineral, the naturally occurring form of sodium nitrate, NaNO3. Chemically it is the sodium analogue of saltpeter. Nitratine crystallizes in the trigonal system, but rarely occurs as well formed crystals. It is isostructural with calcite. It is relatively soft and light with a Mohs hardness of 1.5 to 2 and a specific gravity of 2.24 to 2.29. Its refractive indices are nω=1.587 and nε=1.336.

The Birkeland–Eyde process was one of the competing industrial processes in the beginning of nitrogen-based fertilizer production. It is a multi-step nitrogen fixation reaction that uses electrical arcs to react atmospheric nitrogen (N2) with oxygen (O2), ultimately producing nitric acid (HNO3) with water. The resultant nitric acid was then used as a source of nitrate (NO3−) in the reaction which may take place in the presence of water or another proton acceptor.
Calcium ammonium nitrate or CAN, also known as nitro-limestone or nitrochalk, is a widely used inorganic fertilizer, accounting for 4% of all nitrogen fertilizer used worldwide in 2007.

Ammonia pollution is pollution by the chemical ammonia (NH3) – a compound of nitrogen and hydrogen which is a byproduct of agriculture and industry. Common forms include air pollution by the ammonia gas emitted by rotting agricultural slurry and fertilizer factories while natural sources include the burning coal mines of Jharia, the caustic Lake Natron and the guano of seabird colonies. Gaseous ammonia reacts with other pollutants in the air to form fine particles of ammonium salts, which affect human breathing. Ammonia gas can also affect the chemistry of the soil on which it settles and will, for example, degrade the conditions required by the sphagnum moss and heathers of peatland.



















