Related Research Articles

The endomembrane system is composed of the different membranes (endomembranes) that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that forms a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of plastids or mitochondria, but might have evolved partially from the actions of the latter.

Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.

A lysosome is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane proteins, and its lumenal proteins. The lumen's pH (~4.5–5.0) is optimal for the enzymes involved in hydrolysis, analogous to the activity of the stomach. Besides degradation of polymers, the lysosome is involved in various cell processes, including secretion, plasma membrane repair, apoptosis, cell signaling, and energy metabolism.

Exocytosis is a form of active transport and bulk transport in which a cell transports molecules out of the cell. As an active transport mechanism, exocytosis requires the use of energy to transport material. Exocytosis and its counterpart, endocytosis, are used by all cells because most chemical substances important to them are large polar molecules that cannot pass through the hydrophobic portion of the cell membrane by passive means. Exocytosis is the process by which a large amount of molecules are released; thus it is a form of bulk transport. Exocytosis occurs via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structure at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. This process is in contrast to passive transport, which allows molecules or ions to move down their concentration gradient, from an area of high concentration to an area of low concentration, without energy.
In biology, caveolae, which are a special type of lipid raft, are small invaginations of the plasma membrane in the cells of many vertebrates. They are the most abundant surface feature of many vertebrate cell types, especially endothelial cells, adipocytes and embryonic notochord cells. They were originally discovered by E. Yamada in 1955.

Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus.
Calcium release-activated channels (CRAC) are specialized plasma membrane Ca2+ ion channels. When calcium ions (Ca2+) are depleted from the endoplasmic reticulum (a major store of Ca2+) of mammalian cells, the CRAC channel is activated to slowly replenish the level of calcium in the endoplasmic reticulum. The Ca2+ Release-activated Ca2+ (CRAC) Channel (CRAC-C) Family (TC# 1.A.52) is a member of the Cation Diffusion Facilitator (CDF) Superfamily. These proteins typically have between 4 and 6 transmembrane α-helical spanners (TMSs). The 4 TMS CRAC channels arose by loss of 2TMSs from 6TMS CDF carriers, an example of 'reverse' evolution'.
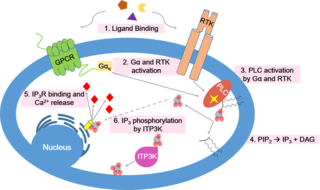
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for cellular signalling, for once it enters the cytosol of the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins. Ca2+ can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as G protein-coupled receptors.
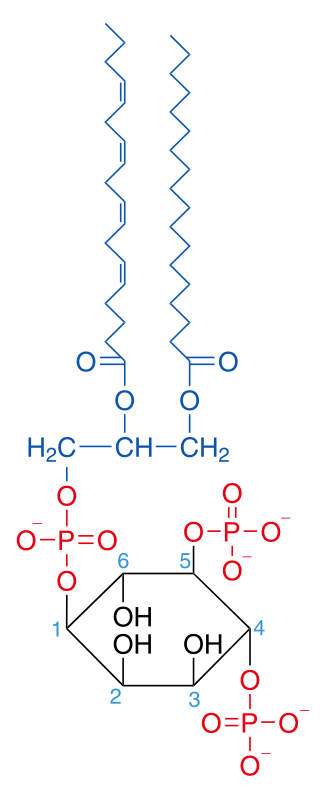
Phosphatidylinositol 4,5-bisphosphate or PtdIns(4,5)P2, also known simply as PIP2 or PI(4,5)P2, is a minor phospholipid component of cell membranes. PtdIns(4,5)P2 is enriched at the plasma membrane where it is a substrate for a number of important signaling proteins. PIP2 also forms lipid clusters that sort proteins.

Synaptotagmins (SYTs) constitute a family of membrane-trafficking proteins that are characterized by an N-terminal transmembrane region (TMR), a variable linker, and two C-terminal C2 domains - C2A and C2B. There are 17 isoforms in the mammalian synaptotagmin family. There are several C2-domain containing protein families that are related to synaptotagmins, including transmembrane (Ferlins, Extended-Synaptotagmin (E-Syt) membrane proteins, and MCTPs) and soluble (RIMS1 and RIMS2, UNC13D, synaptotagmin-related proteins and B/K) proteins. The family includes synaptotagmin 1, a Ca2+ sensor in the membrane of the pre-synaptic axon terminal, coded by gene SYT1.
Phospholipase D (EC 3.1.4.4, lipophosphodiesterase II, lecithinase D, choline phosphatase, PLD; systematic name phosphatidylcholine phosphatidohydrolase) is an enzyme of the phospholipase superfamily that catalyses the following reaction
Cell physiology is the biological study of the activities that take place in a cell to keep it alive. The term physiology refers to normal functions in a living organism. Animal cells, plant cells and microorganism cells show similarities in their functions even though they vary in structure.
When molecules on the surface of a motile eukaryotic cell are crosslinked, they are moved to one end of the cell to form a "cap". This phenomenon, the process of which is called cap formation, was discovered in 1971 on lymphocytes and is a property of amoebae and all locomotory animal cells except sperm. The crosslinking is most easily achieved using a polyvalent antibody to a surface antigen on the cell. Cap formation can be visualised by attaching a fluorophore, such as fluorescein, to the antibody.

PIKfyve, a FYVE finger-containing phosphoinositide kinase, is an enzyme that in humans is encoded by the PIKFYVE gene.

-Cytosis is a suffix that either refers to certain aspects of cells ie cellular process or phenomenon or sometimes refers to predominance of certain type of cells. It essentially means "of the cell". Sometimes it may be shortened to -osis and may be related to some of the processes ending with -esis or similar suffixes.
Vesicle fusion is the merging of a vesicle with other vesicles or a part of a cell membrane. In the latter case, it is the end stage of secretion from secretory vesicles, where their contents are expelled from the cell through exocytosis. Vesicles can also fuse with other target cell compartments, such as a lysosome. Exocytosis occurs when secretory vesicles transiently dock and fuse at the base of cup-shaped structures at the cell plasma membrane called porosome, the universal secretory machinery in cells. Vesicle fusion may depend on SNARE proteins in the presence of increased intracellular calcium (Ca2+) concentration.
An autophagosome is a spherical structure with double layer membranes. It is the key structure in macroautophagy, the intracellular degradation system for cytoplasmic contents. After formation, autophagosomes deliver cytoplasmic components to the lysosomes. The outer membrane of an autophagosome fuses with a lysosome to form an autolysosome. The lysosome's hydrolases degrade the autophagosome-delivered contents and its inner membrane.
Gillian Griffiths, FMedSci FRS is a British cell biologist and immunologist. Griffiths was one of the first to show that immune cells have specialised mechanisms of secretion, and identified proteins and mechanisms that control cytotoxic T lymphocyte secretion. Griffiths is Professor of Cell Biology and Immunology at the University of Cambridge and is the Director of the Cambridge Institute for Medical Research.
Microautophagy is one of the three common forms of autophagic pathway, but unlike macroautophagy and chaperone-mediated autophagy, it is mediated—in mammals by lysosomal action or in plants and fungi by vacuolar action—by direct engulfment of the cytoplasmic cargo. Cytoplasmic material is trapped in the lysosome/vacuole by a random process of membrane invagination.
References
- 1 2 3 "Current Lab Members". Andrews Lab - University of Maryland. Retrieved 16 July 2019.
- 1 2 3 4 "Norma Andrews". iBiology. Retrieved 16 July 2019.
- 1 2 "Norma W. Andrews". University of Maryland. Retrieved 16 July 2019.