Glomerella graminicola is an economically important crop parasite affecting both wheat and maize where it causes the plant disease Anthracnose Leaf Blight.
Alternaria alternata is a fungus causing leaf spots, rots, and blights on many plant parts, and other diseases. It is an opportunistic pathogen on over 380 host species of plant.
Alternaria triticina is a fungal plant pathogen that causes leaf blight on wheat. A. triticina is responsible for the largest leaf blight issue in wheat and also causes disease in other major cereal grain crops. It was first identified in India in 1962 and still causes significant yield loss to wheat crops on the Indian subcontinent. The disease is caused by a fungal pathogen and causes necrotic leaf lesions and in severe cases shriveling of the leaves.

Gibberella zeae, also known by the name of its anamorph Fusarium graminearum, is a fungal plant pathogen which causes fusarium head blight (FHB), a devastating disease on wheat and barley. The pathogen is responsible for billions of dollars in economic losses worldwide each year. Infection causes shifts in the amino acid composition of wheat, resulting in shriveled kernels and contaminating the remaining grain with mycotoxins, mainly deoxynivalenol (DON), which inhibits protein biosynthesis; and zearalenone, an estrogenic mycotoxin. These toxins cause vomiting, liver damage, and reproductive defects in livestock, and are harmful to humans through contaminated food. Despite great efforts to find resistance genes against F. graminearum, no completely resistant variety is currently available. Research on the biology of F. graminearum is directed towards gaining insight into more details about the infection process and reveal weak spots in the life cycle of this pathogen to develop fungicides that can protect wheat from scab infection.

Diaporthe helianthi is a fungal pathogen that causes Phomopsis stem canker of sunflowers. In sunflowers, Phomopsis helianthi is the causative agent behind stem canker. Its primary symptom is the production of large canker lesions on the stems of sunflower plants. These lesions can eventually lead to lodging and plant death. This disease has been shown to be particularly devastating in southern and eastern regions of Europe, although it can also be found in the United States and Australia. While cultural control practices are the primary method of controlling for Stem Canker, there have been a few resistant cultivars developed in regions of Europe where the disease is most severe.

Ascochyta is a genus of ascomycete fungi, containing several species that are pathogenic to plants, particularly cereal crops. The taxonomy of this genus is still incomplete. The genus was first described in 1830 by Marie-Anne Libert, who regarded the spores as minute asci and the cell contents as spherical spores. Numerous revisions to the members of the genus and its description were made for the next several years. Species that are plant pathogenic on cereals include, A. hordei, A. graminea, A. sorghi, A. tritici. Symptoms are usually elliptical spots that are initially chlorotic and later become a necrotic brown. Management includes fungicide applications and sanitation of diseased plant tissue debris.
Cercospora arachidicola is a fungal ascomycete plant pathogen that causes early leaf spot of peanut. Peanuts originated in South America and are cultivated globally in warm, temperate and tropical regions.
Alternaria dauci is a plant pathogen. The English name of the disease it incites is "carrot leaf blight".

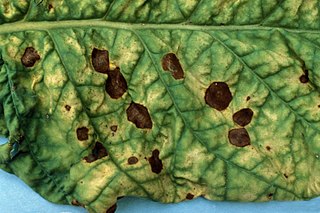
Alternaria solani is a fungal pathogen that produces a disease in tomato and potato plants called early blight. The pathogen produces distinctive "bullseye" patterned leaf spots and can also cause stem lesions and fruit rot on tomato and tuber blight on potato. Despite the name "early," foliar symptoms usually occur on older leaves. If uncontrolled, early blight can cause significant yield reductions. Primary methods of controlling this disease include preventing long periods of wetness on leaf surfaces and applying fungicides. Early blight can also be caused by Alternaria tomatophila, which is more virulent on stems and leaves of tomato plants than Alternaria solani.

Ascochyta pisi is a fungal plant pathogen that causes ascochyta blight on pea, causing lesions of stems, leaves, and pods. These same symptoms can also be caused by Ascochyta pinodes, and the two fungi are not easily distinguishable.

Didymella bryoniae, syn. Mycosphaerella melonis, is an ascomycete fungal plant pathogen that causes gummy stem blight on the family Cucurbitaceae, which includes cantaloupe, cucumber, muskmelon and watermelon plants. The anamorph/asexual stage for this fungus is called Phoma cucurbitacearum. When this pathogen infects the fruit of cucurbits it is called black rot.
Peronosclerospora sorghi is a plant pathogen. It is the causal agent of sorghum downy mildew. The pathogen is a fungal-like protist in the oomycota, or water mold, class. Peronosclerospora sorghi infects susceptible plants though sexual oospores, which survive in the soil, and asexual sporangia which are disseminated by wind. Symptoms of sorghum downy mildew include chlorosis, shredding of leaves, and death. Peronosclerospora sorghi infects maize and sorghum around the world, but causes the most severe yield reductions in Africa. The disease is controlled mainly through genetic resistance, chemical control, crop rotation, and strategic timing of planting.

Cercospora sojina is a fungal plant pathogen which causes frogeye leaf spot of soybeans. Frog eye leaf spot is a major disease on soybeans in the southern U.S. and has recently started to expand into the northern U.S. where soybeans are grown. The disease is also found in other soybean production areas of the world.

Cercospora melongenae is a fungal plant pathogen that causes leaf spot on eggplant. It is a deuteromycete fungus that is primarily confined to eggplant species. Some other host species are Solanum aethiopicum and Solanum incanum. This plant pathogen only attacks leaves of eggplants and not the fruit. It is fairly common among the fungi that infect community gardens and home gardens of eggplant. Generally speaking, Cercospora melongenae attacks all local varieties of eggplants, but is most severe on the Philippine eggplant and less parasitic on a Siamese variety.
This article summarizes different crops, what common fungal problems they have, and how fungicide should be used in order to mitigate damage and crop loss. This page also covers how specific fungal infections affect crops present in the United States.

Grey leaf spot (GLS) is a foliar fungal disease that affects maize, also known as corn. GLS is considered one of the most significant yield-limiting diseases of corn worldwide. There are two fungal pathogens that cause GLS: Cercospora zeae-maydis and Cercospora zeina. Symptoms seen on corn include leaf lesions, discoloration (chlorosis), and foliar blight. Distinct symptoms of GLS are rectangular, brown to gray necrotic lesions that run parallel to the leaf, spanning the spaces between the secondary leaf veins. The fungus survives in the debris of topsoil and infects healthy crops via asexual spores called conidia. Environmental conditions that best suit infection and growth include moist, humid, and warm climates. Poor airflow, low sunlight, overcrowding, improper soil nutrient and irrigation management, and poor soil drainage can all contribute to the propagation of the disease. Management techniques include crop resistance, crop rotation, residue management, use of fungicides, and weed control. The purpose of disease management is to prevent the amount of secondary disease cycles as well as to protect leaf area from damage prior to grain formation. Corn grey leaf spot is an important disease of corn production in the United States, economically significant throughout the Midwest and Mid-Atlantic regions. However, it is also prevalent in Africa, Central America, China, Europe, India, Mexico, the Philippines, northern South America, and Southeast Asia. The teleomorph of Cercospora zeae-maydis is assumed to be Mycosphaerella sp.

Ascochyta blights occur throughout the world and can be of significant economic importance. Three fungi contribute to the ascochyta blight disease complex of pea. Ascochyta pinodes causes Mycosphaerella blight. Ascochyta pinodella causes Ascochyta foot rot, and Ascochyta pisi causes Ascochyta blight and pod spot. Of the three fungi, Ascochyta pinodes is of the most importance. These diseases are conducive under wet and humid conditions and can cause a yield loss of up to fifty percent if left uncontrolled. The best method to control ascochyta blights of pea is to reduce the amount of primary inoculum through sanitation, crop-rotation, and altering the sowing date. Other methods—chemical control, biological control, and development of resistant varieties—may also be used to effectively control ascochyta diseases.

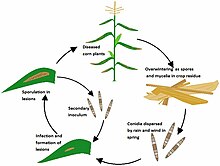
Southern corn leaf blight (SCLB) is a fungal disease of maize caused by the plant pathogen Bipolaris maydis.

Cherry leaf spot is a fungal disease which infects cherries and plums. Sweet, sour, and ornamental cherries are susceptible to the disease, being most prevalent in sour cherries. The variety of sour cherries that is the most susceptible are the English morello cherries. This is considered a serious disease in the Midwest, New England states, and Canada. It has also been estimated to infect 80 percent of orchards in the Eastern states. It must be controlled yearly to avoid a significant loss of the crop. If not controlled properly, the disease can dramatically reduce yields by nearly 100 percent. The disease is also known as yellow leaf or shothole disease to cherry growers due to the characteristic yellowing leaves and shot holes present in the leaves upon severe infection.

Alternaria brassicicola is a fungal necrotrophic plant pathogen that causes black spot disease on a wide range of hosts, particularly in the genus of Brassica, including a number of economically important crops such as cabbage, Chinese cabbage, cauliflower, oilseeds, broccoli and canola. Although mainly known as a significant plant pathogen, it also contributes to various respiratory allergic conditions such as asthma and rhinoconjunctivitis. Despite the presence of mating genes, no sexual reproductive stage has been reported for this fungus. In terms of geography, it is most likely to be found in tropical and sub-tropical regions, but also in places with high rain and humidity such as Poland. It has also been found in Taiwan and Israel. Its main mode of propagation is vegetative. The resulting conidia reside in the soil, air and water. These spores are extremely resilient and can overwinter on crop debris and overwintering herbaceous plants.