
Oxytetracycline is a broad-spectrum tetracycline antibiotic, the second of the group to be discovered.
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.

Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compounds found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid biosynthesis. The enzyme catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone.

(S)-3,5-Dihydroxyphenylglycine or DHPG is a potent agonist of group I metabotropic glutamate receptors (mGluRs) mGluR1 and mGluR5.

Isomigrastatin is an analogue of migrastatin, an organic compound that naturally occurs in the Streptomyces platensis bacteria. Isomigrastatin has shown promise as a drug in the treatment of cancer. A laboratory synthesis was reported in 2007.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.

Macrophomic acid is a fungal metabolite isolated from the fungus Macrophoma commelinae. The enzyme macrophomate synthase converts 5-acetyl-4-methoxy-6-methyl-2-pyrone to 4-acetyl-3-methoxy-5-methyl-benzoic acid through an unusual intermolecular Diels-Alder reaction. The pathway to formation of macrophomic acid suggests that the enzyme is a natural Diels-Alderase. Formation of this type of aromatic ring compound normally proceeds via the shikimate and polyketide pathways; however, the production of macrophomic acid by macrophomate synthase proceeds totally differently. Learning about the production of macrophomic acid by a possible natural Diels-Alderase enzyme is important in understanding enzyme catalytic mechanisms. This knowledge can then be applied to organic synthesis.
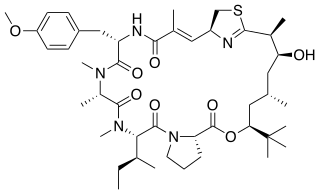
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.

Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5.
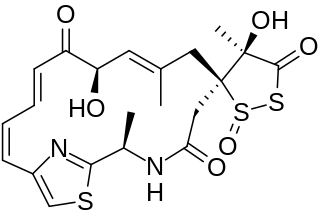
Leinamycin is an 18-membered macrolactam produced by several species of Streptomyces atroolivaceus. This macrolactam has also been shown to exhibit antitumor properties as well as antimicrobial properties against gram-positive and gram-negative bacteria. The presence of a spiro-fused 1,3-dioxo-1,2-dithiolane moiety was a unique structural property at the time of this compound's discovery and it plays an important role in leinamycin's antitumor and antibacterial properties due to its ability to inhibit DNA synthesis.
Curacin A is a hybrid polyketide synthase (PKS)/nonribosomal peptide synthase (NRPS) derived natural product produced isolated from the cyanobacterium Lyngbya majuscula. Curacin A belongs to a family of natural products including jamaicamide, mupirocin, and pederin that have an unusual terminal alkene. Additionally, Curacin A contains a notable thiazoline ring and a unique cyclopropyl moiety, which is essential to the compound's biological activity. Curacin A has been characterized as potent antiproliferative cytotoxic compound with notable anticancer activity for several cancer lines including renal, colon, and breast cancer. Curacin A has been shown to interact with colchicine binding sites on tubulin, which inhibits microtubule polymerization, an essential process for cell division and proliferation.

Portentol is a complex polyketide first isolated in 1967 from the lichen Roccella portentosa and has since been extracted from various other lichen. It has exhibited moderate activity toward several cancer cell lines. Of greater interest is the structural skeleton and chemical space the molecule possesses, which were ultimately determined by detailed NMR studies and X-ray analysis. The spiro tricyclic core contains nine consecutive stereocenters, two of which are adjacent quaternary centers, and a β-keto-δ-lactone moiety. The densely functionalized natural product offered an exciting challenge to total synthesis.

BY1 is a taxonomically unidentified basidiomycete fungus. ITS sequencing has placed it in the Russulales and is referred to as a stereaceous basidiomycete. Chemotaxonomically supporting its placement in this group, it produces fomannoxins and vibralactones. The fungus' mycelia were isolated from dead aspen in Minnesota, USA. It is presumed to decompose wood by white rot.
Fostriecin is a type I polyketide synthase (PKS) derived natural product, originally isolated from the soil bacterium Streptomyces pulveraceus. It belongs to a class of natural products which characteristically contain a phosphate ester, an α,β-unsaturated lactam and a conjugated linear diene or triene chain produced by Streptomyces. This class includes structurally related compounds cytostatin and phoslactomycin. Fostriecin is a known potent and selective inhibitor of protein serine/threonine phosphatases, as well as DNA topoisomerase II. Due to its activity against protein phosphatases PP2A and PP4 which play a vital role in cell growth, cell division, and signal transduction, fostriecin was looked into for its antitumor activity in vivo and showed in vitro activity against leukemia, lung cancer, breast cancer, and ovarian cancer. This activity is thought to be due to PP2A's assumed role in regulating apoptosis of cells by activating cytotoxic T-lymphocytes and natural killer cells involved in tumor surveillance, along with human immunodeficiency virus-1 (HIV-1) transcription and replication.

Borrelidin is an 18-membered polyketide macrolide derived from several Streptomyces species. First discovered in 1949 from Streptomyces rochei, Borrelidin shows antibacterial activity by acting as an inhibitor of threonyl-tRNA synthetase and features a nitrile moiety, a unique functionality in natural products., Borrelidin also exhibits potent angiogenesis inhibition, which was shown in a rat aorta matrix model. Other studies have been performed to show that low concentrations of borrelidin can suppress growth and induce apoptosis in malignant acute lymphoblastic leukemia cells. Borredlidin's antimalarial activity has also been shown in vitro and in vivo.

Swinholides are dimeric 42 carbon-ring polyketides that exhibit a 2-fold axis of symmetry. Found mostly in the marine sponge Theonella, swinholides encompass cytotoxic and antifungal activities via disruption of the actin skeleton. Swinholides were first described in 1985 and the structure and stereochemistry were updated in 1989 and 1990, respectively. Thirteen swinholides have been described in the literature, including close structural compounds such as misakinolides/bistheonellides, ankaraholides, and hurgholide A It is suspected that symbiotic microbes that inhabit the sponges rather than the sponges themselves produce swinholides since the highest concentration of swinholides are found in the unicellular bacterial fraction of sponges and not in the sponge fraction or cyanobacteria fraction that also inhabit the sponges.
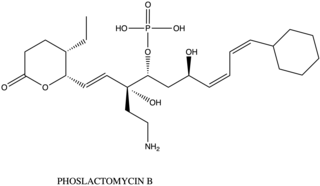
Phoslactomycin (PLM) is a natural product from the isolation of Streptomyces species. This is an inhibitor of the protein serine/threonine phosphatase which is the protein phosphate 2A (PP2A). The PP2A involves the growth factor of the cell such as to induce the formation of mitogen-activated protein interaction and playing a role in cell division and signal transduction. Therefore, PLM is used for the drug that prevents the tumor, cancer, or bacteria. There are nowsaday has 7 kinds of different PLM from PLM A to PLM G which differ the post-synthesis from the biosynthesis of PLM.















