Apples

Spraying for control of fungal diseases of apples started in the U.S. some time between 1880 and 1905.
Apple scab
Apple scab is caused by the fungus Venturia inaequalis . Mating among different strains of the fungus occurs shortly after leaf fall with spores developing in the fallen leaves during the winter. Spring rains cause spores to be forcibly discharged; they can be carried long distances by air currents to flowers, leaves, or young fruit. [13] The spores then continue to develop and are released over a period of 5–9 weeks. [14] These spores germinate and penetrate the outer layers of the plant, causing infection. The fungus grows beneath the cuticle and eventually ruptures to form dark green lesions. The number of lesions per leaf can be as few as 1–2 or there can be hundreds. [14] The USDA's National Agricultural Pesticide Impact Assessment Program estimated that 100% of eastern apple orchards are stricken with apple scab and that without fungicide treatments yield losses would be as high as 90%. [15] In the western orchards 52% are infected and apple yield loss can be as high as 22%. [11] However, with the use of fungicides experiments have shown heavy reductions in the percentage of infected apples. One study was able to reduce the incidence from 77% to 2%. [16]
Bitter rot
Bitter rot is a major disease in the Southeast U.S. in the summer months when the weather is hot and damp. [17] The organism has a short incubation period and as a result epidemics of bitter rot can develop rapidly. The presence of the disease is first indicated by the very small light brown sunken spots beneath the apple skin. [18] As the fungus grows and invades more of the apple tissue, the area becomes engorged until the entire apple is rotted. [19] Around the start of the 20th century Bordeaux mixture was the primary technique for controlling the disease; in the 1940s growers shifted to synthetic chemical use. [18] [19] Without these fungicides is it estimated that apple yield loss due to bitter rot would be as high at 90%. [15] The most effective fungicides for bitter rot control include the multisite mode-of-action fungicide captan, the osmotic signal transduction disrupter fludioxonil, the oxidative phosphorylation uncoupler fluazinam, the QOI inhibitors pyraclostrobin and trifloxystrobin, and the succinate dehydrogenase inhibitor (SDHI) benzovindiflupyr. [20]
Black pox
This disease primarily affects the southeastern portion of the United States and is known to infect the cultivars Rome Beauty, Grimes Golden, Delicious, York Imperial, and Golden Delicious. Lesions on twigs are well defined, conical, shiny black swellings and on the fruit itself they are black, spherical, slightly sunken spots. Severely affected leaves may die within 2 to 3 weeks of infection. Infected branches will grow poorly, lose their leaves early, and die. Black pox can be controlled with the same fungicide sprays that are used to treat scab. [14]
Blossom-end rot
The first symptom of blossom-end rot is soft, wet, and reddish discolorations that appear in the late summer months. As the rot stops growing it will begin to dry out and appear sunken. [14] The affected fruit will often drop prematurely. Fungicide experiments have shown reduced incidence, from 5% to less than 0.5%, with treatment. [21]
Brooks fruit spot
Brooks fruit spot is a minor disease of the apple and is found mostly throughout the Northeast and Mid-Atlantic regions. As the fruit first emerges the disease appears as dark green lesions on the fruit of the apple. As the apple begins to emerge these blemishes grow and change to purple or green. [14] Brooks fruit spot is usually controlled with fungicides applied during the early cover-spray period. One study showed that apple orchards that were not treated with fungicides had 87% of their fruit infected with brooks fruit spot while only 1–6% of trees that were treated with fungicides showed symptoms of the disease. [22]
Fire blight
Fire blight was first described in New York in the late 18th century, and moved west with settlers, becoming established throughout North American apple production areas by the early 20th century. While fire blight has always been a concern in eastern apple production, severe outbreaks in the west in recent years have caused growers there to adopt more consistent and vigorous monitoring and management programs as well. [23] The disease can affect every part of the tree, from the fruit to the trunk. [14] Infected trees may die within months or can linger for years with severely reduced yields. In addition to the scorched appearance of the plant parts that gives the disease its name, plant tissues infected with the bacteria will exude milky or reddish-brown ooze. Initially copper sprays were used for fire blight control in the 1930s but this method had limited success. [24] In the 1950s streptomycin and oxytetracycline showed high success in controlling fire blight in comparison to copper. Since then streptomycin sprayed two to three times during blooming phase has become the treatment of choice.
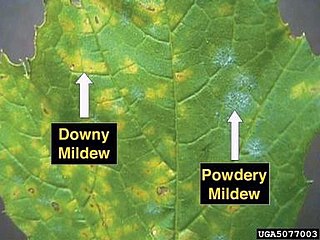
Powdery mildew
Powdery mildew is a common fungal infection of apples and can occur in almost any apple growing climate. The spores from fungi preserved over the winter are released from the unfolding leaves of the buds. The spores, carried by the wind, infect leaves, blossoms, and fruit. [14] The fungus spreads until it covers the whole leaf and then grows down twigs, covering them with a gray felt. This results in aborted blossoms, reduced finish quality, and reduced yield. [25] The USDA has estimated that 40% of apple orchards in the East and 50% of apple orchards in the West are infected with powdery mildew. [11] Without control of the fungus yields would drop by 65%. [15]
Quince rust
Quince rust infects the fruit of the apple trees but does not affect the leaves. Quince rust spores infect cedar trees and create cylindrical galls from which emerge spore horns the following spring. These galls may produce spores for up to twenty years. Quince rust is economically important primarily when an extended wetting period with a mean temperature above 10 °C (50 °F) occurs between the tight cluster and late pink bud stages. Under these conditions, economic losses may occur throughout large geographic areas. [14] Experiments with fungicide sprays have been shown to provide complete control of rust. [26]
White rot
White rot gets its name from the soft, watery, and light-colored rotted fruit that is left over after an apple is infected. The fungus survives from season to season in the dead bark and mummified fruit of the apple tree. Spores can survive in the dead bark for up to six years. [27] Growers are advised to treat for white rot once the sugar content of the fruit reaches approximately 10%. USDA estimates that 20% of apple orchards in eastern states are infected with the fungus and that without fungicide use yield losses would be 65%. [15]