
A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.
Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health, but cannot synthesize them.

α-Linolenic acid, also known as alpha-linolenic acid (ALA), is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.
gamma-Linolenic acid or GLA is an n−6, or omega-6, fatty acid found primarily in seed oils. When acting on GLA, arachidonate 5-lipoxygenase produces no leukotrienes and the conversion by the enzyme of arachidonic acid to leukotrienes is inhibited.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.

Calendula officinalis, the pot marigold, common marigold, ruddles, Mary's gold or Scotch marigold, is a flowering plant in the daisy family Asteraceae. It is probably native to southern Europe, though its long history of cultivation makes its precise origin unknown, and it may possibly be of garden origin. It is also widely naturalised farther north in Europe and elsewhere in warm temperate regions of the world.
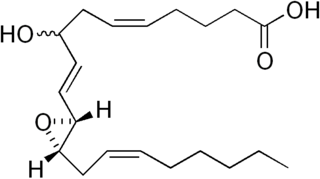
Hepoxilins (Hx) are a set of epoxyalcohol metabolites of polyunsaturated fatty acids (PUFA), i.e. they possess both an epoxide and an alcohol residue. HxA3, HxB3, and their non-enzymatically formed isomers are nonclassic eicosanoid derived from acid the (PUFA), arachidonic acid. A second group of less well studied hepoxilins, HxA4, HxB4, and their non-enzymatically formed isomers are nonclassical eicosanoids derived from the PUFA, eicosapentaenoic acid. Recently, 14,15-HxA3 and 14,15-HxB3 have been defined as arachidonic acid derivatives that are produced by a different metabolic pathway than HxA3, HxB3, HxA4, or HxB4 and differ from the aforementioned hepoxilins in the positions of their hydroxyl and epoxide residues. Finally, hepoxilin-like products of two other PUFAs, docosahexaenoic acid and linoleic acid, have been described. All of these epoxyalcohol metabolites are at least somewhat unstable and are readily enzymatically or non-enzymatically to their corresponding trihydroxy counterparts, the trioxilins (TrX). HxA3 and HxB3, in particular, are being rapidly metabolized to TrXA3, TrXB3, and TrXC3. Hepoxilins have various biological activities in animal models and/or cultured mammalian tissues and cells. The TrX metabolites of HxA3 and HxB3 have less or no activity in most of the systems studied but in some systems retain the activity of their precursor hepoxilins. Based on these studies, it has been proposed that the hepoxilins and trioxilins function in human physiology and pathology by, for example, promoting inflammation responses and dilating arteries to regulate regional blood flow and blood pressure.
Calendic acid is an unsaturated fatty acid, named for the pot marigold, from which it is obtained. It is chemically similar to the conjugated linoleic acids; laboratory work suggests it may have similar in vitro bioactivities.

α-Eleostearic acid or (9Z,11E,13E)-octadeca-9,11,13-trienoic acid, is an organic compound, a conjugated fatty acid and one of the isomers of octadecatrienoic acid. It is often called simply eleostearic acid although there is also a β-eleostearic acid. Its high degree of unsaturation gives tung oil its properties as a drying oil.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

α-Parinaric acid is a conjugated polyunsaturated fatty acid. Discovered by Tsujimoto and Koyanagi in 1933, it contains 18 carbon atoms and 4 conjugated double bonds. The repeating single bond-double bond structure of α-parinaric acid distinguishes it structurally and chemically from the usual "methylene-interrupted" arrangement of polyunsaturated fatty acids that have double-bonds and single bonds separated by a methylene unit (−CH2−). Because of the fluorescent properties conferred by the alternating double bonds, α-parinaric acid is commonly used as a molecular probe in the study of biomembranes.
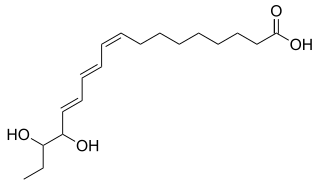
15,16-Dihydroxy-α-eleostearic acid, or 15,16-Dihydroxy-(9Z,11E,13E)-9,11,13-octadecatrienoic acid, is an organic compound with formula C
18H
30O
4, or H3C-CH2-(-CH(OH)-)2(-CH=CH-)3-(-CH2-)7-(C=O)OH. It can be seen as derived from α-eleostearic acid by the replacement of two hydrogen atoms by two hydroxyl (OH) groups.
β-Eleostearic acid, or (9E,11E,13E)-octadeca-9,11,13-trienoic acid, is an organic compound with formula C
18H
30O
2 or H3C(CH2)3(CH=CH)3(CH2)7COOH. It is an all-trans isomer of octadecatrienoic acid.
Eleostearic acid is a fatty acid, one of two isomers of octadecatrienoic acid:
Protectin D1 also known as neuroprotectin D1 and abbreviated most commonly as PD1 or NPD1 is a member of the class of specialized proresolving mediators. Like other members of this class of polyunsaturated fatty acid metabolites, it possesses strong anti-inflammatory, anti-apoptotic and neuroprotective activity. PD1 is an aliphatic acyclic alkene 22 carbons in length with two hydroxyl groups at the 10 and 17 carbon positions and one carboxylic acid group at the one carbon position.
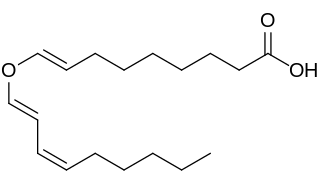
Divinylether fatty acids contain a fatty acid chemically combined with a doubly unsaturated carbon chain linked by an oxygen atom (ether). Fatty acid hydroperoxides generated by plant lipoxygenases from linoleic and linolenic acids are known to serve as substrates for a divinyl ether synthase which produces divinyl ether fatty acids. Up to date divinyl ethers were detected only within the plant kingdom.
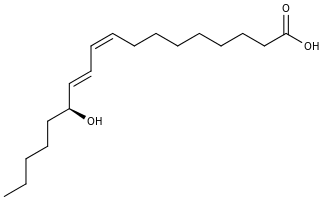
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.
Dihydroxy-E,Z,E-PUFA are metabolites of polyunsaturated fatty acids (PUFA) that possess two hydroxyl residues and three in series conjugated double bonds having the E,Z,E cis-trans configuration. These recently classified metabolites are distinguished from the many other dihydroxy-PUFA with three conjugated double bonds that do not have this critical E,Z,E configuration: they inhibit the function of platelets and therefore may be involved in controlling and prove useful for inhibiting human diseases which involve the pathological activation of these blood-borne elements.
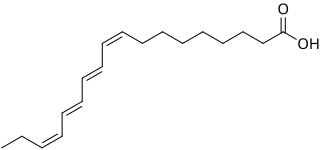
An octadecatetraenoic acid is a chemical compound with formula C
18H
28O
2, a polyunsaturated fatty acid with whose molecule has an 18-carbon unbranched backbone with four double bonds.











