
Joubert syndrome is a rare autosomal recessive genetic disorder that affects the cerebellum, an area of the brain that controls balance and coordination.
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31.
Adams–Oliver syndrome (AOS) is a rare congenital disorder characterized by defects of the scalp and cranium, transverse defects of the limbs, and mottling of the skin.

Cohen syndrome is a very rare autosomal recessive genetic disorder with varied expression, characterised by obesity, intellectual disability, distinct craniofacial abnormalities and potential ocular dysfunction.

Iduronate 2-sulfatase is a sulfatase enzyme associated with Hunter syndrome. It catalyses hydrolysis of the 2-sulfate groups of the L-iduronate 2-sulfate units of dermatan sulfate, heparan sulfate and heparin.
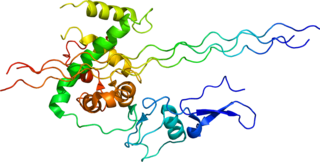
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.

Hay–Wells syndrome is one of at least 150 known types of ectodermal dysplasia. These disorders affect tissues that arise from the ectodermal germ layer, such as skin, hair, and nails.

Yunis–Varon syndrome (YVS), also called cleidocranial dysplasia with micrognathia or absent thumbs and distal aphalangia, is an extremely rare autosomal recessive multisystem congenital disorder which affects the skeletal system, ectodermal tissue, heart and respiratory system. It was first described by Emilio Yunis and Humberto Váron from the National University of Colombia.
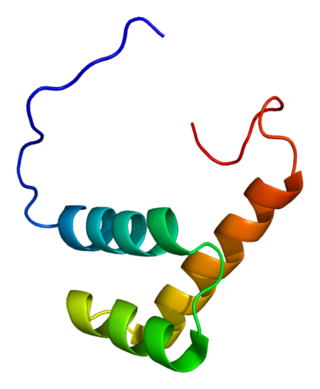
Zinc finger E-box-binding homeobox 2 is a protein that in humans is encoded by the ZEB2 gene. The ZEB2 protein is a transcription factor that plays a role in the transforming growth factor β (TGFβ) signaling pathways that are essential during early fetal development.

Intermembrane lipid transfer protein VPS13B, also known as vacuolar protein sorting-associated 13B, and Cohen syndrome protein 1 is a protein that in humans is encoded by the VPS13B gene. It is a giant protein associated with the Golgi apparatus that is believed to be involved in post-Golgi apparatus sorting and trafficking. Mutations in the human VPS13B gene cause Cohen syndrome.

Endothelin receptor type B, (ET-B) is a protein that in humans is encoded by the EDNRB gene.

Carbonic anhydrase 12 is an enzyme that in humans is encoded by the CA12 gene.

Sal-like 1 (Drosophila), also known as SALL1, is a protein which in humans is encoded by the SALL1 gene. As the full name suggests, it is one of the human versions of the spalt (sal) gene known in Drosophila.

Visual system homeobox 2 is a protein that in humans is encoded by the VSX2 gene.
Germline mosaicism, also called gonadal mosaicism, is a type of genetic mosaicism where more than one set of genetic information is found specifically within the gamete cells; conversely, somatic mosaicism is a type of genetic mosaicism found in somatic cells. Germline mosaicism can be present at the same time as somatic mosaicism or individually, depending on when the conditions occur. Pure germline mosaicism refers to mosaicism found exclusively in the gametes and not in any somatic cells. Germline mosaicism can be caused either by a mutation that occurs after conception, or by epigenetic regulation, alterations to DNA such as methylation that do not involve changes in the DNA coding sequence.

Autoimmune polyendocrine syndrome type 1 (APS-1), is a subtype of autoimmune polyendocrine syndrome. It causes the dysfunction of multiple endocrine glands due to autoimmunity. It is a genetic disorder, inherited in autosomal recessive fashion due to a defect in the AIRE gene , which is located on chromosome 21 and normally confers immune tolerance.

Vacuolar protein sorting 53 homolog is a protein that in humans is encoded by the VPS53 gene.

Ubiquinol-cytochrome c reductase, complex III subunit VII, 9.5kDa is a protein that in humans is encoded by the UQCRQ gene. This ubiqinone-binding protein is a subunit of mitochondrial Complex III in the electron transport chain. A mutation in the UQCRQ gene has been shown to cause severe neurological disorders. Infection by Trypanosoma cruzi can cause oxidative modification of this protein in cardiac muscle tissue.

Birk-Barel syndrome is a rare genetic disorder associated with the KCNK9 gene. Signs and symptoms include mental retardation, hypotonia, hyperactivity, and syndromic facies.
Halperin-Birk syndrome (HLBKS) is a rare autosomal recessive neurodevelopmental disorder caused by a null mutation in the SEC31A gene. Signs and symptoms include intrauterine growth retardation, marked developmental delay, spastic quadriplegia with profound contractures, dysmorphism, and optic nerve atrophy with no eye fixation. Brain MRI demonstrated microcephaly and agenesis of the corpus callosum.













