
Urology, also known as genitourinary surgery, is the branch of medicine that focuses on surgical and medical diseases of the urinary system and the reproductive organs. Organs under the domain of urology include the kidneys, adrenal glands, ureters, urinary bladder, urethra, and the male reproductive organs.

Chandler Park is an American physician, medical journalist, and clinical researcher. In June 2021, his cancer research was published in prominent medical journals including the New England Journal of Medicine and Journal of Clinical Oncology. Park also contributes regularly as an expert physician for popular newspapers and magazines such as Newsweek, Reader's Digest, U.S. News & World Report, The Exponent-Telegram, College of St. Scholastica, and Medscape and writes medical news for Doximity.

Andrew C. von Eschenbach was the Commissioner of the United States Food and Drug Administration from 2006 to 2009. He became acting Commissioner on September 26, 2005, after the resignation of his predecessor Lester Crawford, and was confirmed as Commissioner by the Senate on December 7, 2006. He was previously the 12th director of the National Cancer Institute.
Nicholas J. Vogelzang was a medical oncologist with Comprehensive Cancer Centers of Nevada (CCCN). He serves as medical director of the Research Executive Committee and Associate Chair of the Developmental Therapeutics and Genitourinary Committees for US Oncology Research. His research interests include clinical trials for genitourinary malignancies and mesothelioma.
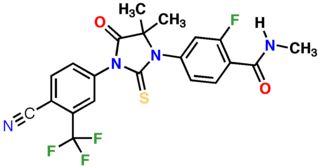
Enzalutamide, sold under the brand name Xtandi, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is indicated for use in conjunction with castration in the treatment of metastatic castration-resistant prostate cancer (mCRPC), nonmetastatic castration-resistant prostate cancer, and metastatic castration-sensitive prostate cancer (mCSPC). It is taken by mouth.
James L. Gulley is an American cancer researcher and the Director of the Medical Oncology Service at National Cancer Institute.
Treatment for prostate cancer may involve active surveillance, surgery, radiation therapy – including brachytherapy and external-beam radiation therapy, proton therapy, high-intensity focused ultrasound (HIFU), cryosurgery, hormonal therapy, chemotherapy, or some combination. Treatments also extend to survivorship based interventions. These interventions are focused on five domains including: physical symptoms, psychological symptoms, surveillance, health promotion and care coordination. However, a published review has found only high levels of evidence for interventions that target physical and psychological symptom management and health promotion, with no reviews of interventions for either care coordination or surveillance. The favored treatment option depends on the stage of the disease, the Gleason score, and the PSA level. Other important factors include the man's age, his general health, and his feelings about potential treatments and their possible side-effects. Because all treatments can have significant side-effects, such as erectile dysfunction and urinary incontinence, treatment discussions often focus on balancing the goals of therapy with the risks of lifestyle alterations.
Simon J. Hall is an American researcher who is the Associate Professor and Kyung Hyun Kim, M.D. Chair of Urology and Assistant Professor, Department of Gene and Cell Medicine at The Mount Sinai School of Medicine, as well as the Director of the Barbara and Maurice Deane Prostate Health and Research Center at The Mount Sinai Medical Center, both in New York City.
William K. Oh, is an American medical oncologist, academic and industry leader and expert in the management of genitourinary malignancies, including prostate, renal, bladder and testicular cancers.
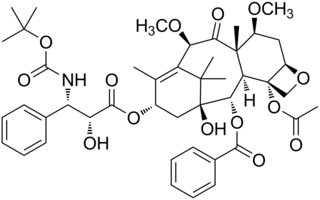
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Dr. Cora Sternberg is an American medical oncologist at Weill Cornell Medicine and NewYork-Presbyterian Hospital, serving as a member of the Genitourinary (GU) Oncology Program. Dr. Sternberg facilitates the continued growth and development of clinical and translational research programs in GU malignancies. Dr. Sternberg is an internationally respected leader in the field of medical oncology and urological malignancies and a recognized expert in the area of new drug development. She is known for her seminal contributions in bladder cancer, her strong track record of sustained genito-urinary (GU) oncology leadership and collaboration in multiple practice-changing clinical trials, including novel medicines, and her current role applying her expertise in oncology and GU cancers to precision medicine to further improve outcomes for patients. Dr. Sternberg has been decidedly influential in the development of novel hormonal therapies and checkpoint inhibitors across the landscape of GU oncology as evidenced in her curriculum vitae. She is a globally respected researcher who has lectured extensively at universities and cancer symposia worldwide (>800). As Clinical Director of the Englander Institute for Precision Medicine (EIPM), Dr. Sternberg develops strategies to incorporate genomic sequencing and precision medicine throughout the Weill Cornell Medicine and NewYork-Presbyterian healthcare network, including Lower Manhattan, Brooklyn and Queens.
Darolutamide, sold under the brand name Nubeqa, is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men. It is specifically approved to treat non-metastatic castration-resistant prostate cancer (nmCRPC) in conjunction with surgical or medical castration. The medication is taken by mouth twice per day with food.

Thomas E. Hutson is an American medical oncologist and cancer researcher based in Dallas, Texas. He is the director of Genitourinary Oncology Program and co-director of the Urologic Cancer Research and Treatment Center at Baylor University Medical Center. He is a Professor of Medicine at the Texas A&M Health Science Center College of Medicine and serves as a chair of Genitourinary Research for US Oncology and McKesson.
Deborah Watkins Bruner is an American researcher, clinical trialist, and academic. She is the senior vice president for research at Emory University. Her research focus is on patient reported outcomes, symptom management across cancer sites, sexuality after cancer treatment, and effectiveness of radiotherapy modalities. Bruner's research has been continually funding since 1998, with total funding of her research exceeding $180 million. She is ranked among the top five percent of all National Institutes of Health-funded investigators worldwide since 2012, according to the Blue Ridge Institute for Medical Research.

Philip W. Kantoff is a medical oncologist. He is the chairman and chief executive officer (CEO) of Convergent Therapeutics. He served as the Chairman of Medicine at Memorial Sloan Kettering Cancer Center between 2015 and 2021. He is best known for his contributions to the impact of DNA abnormalities in prostate cancer and the discovery of therapies for metastatic hormone-sensitive prostate cancer.

Andrea B. Apolo is an American medical oncologist specialized in bladder cancer research. She is an investigator in the National Cancer Institute's genitourinary malignancies branch and head of the bladder cancer section.
Karen E. Knudsen is Chief Executive Officer of American Cancer Society and its advocacy affiliate the American Cancer Society Cancer Action Network. She is the first woman to hold that position in either organization.
Elizabeth R. Plimack is an American medical oncologist. She is a professor in the Department of Hematology/Oncology and Chief of the Division of Genitourinary Medical Oncology at the Fox Chase Cancer Center. In these roles, she researches the treatment of genitourinary malignancies with a focus on bladder and kidney cancers.
Ronald de Wit is a professor of medical oncology at Erasmus University Medical Center in Rotterdam, Netherlands. He is the founding chairman of the Dutch Uro-Oncology Study Group (DUOS).







