
Halteres are a pair of small club-shaped organs on the body of two orders of flying insects that provide information about body rotations during flight. Insects of the large order Diptera (flies) have halteres which evolved from a pair of ancestral hindwings, while males of the much smaller order Strepsiptera (stylops) have halteres which evolved from a pair of ancestral forewings.

Drosophila melanogaster is a species of fly in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly", or "banana fly". In the wild, D. melanogaster are attracted to rotting fruit and fermenting beverages, and are often found in orchards, kitchens and pubs.

The visual system is the physiological basis of visual perception. The system detects, transduces and interprets information concerning light within the visible range to construct an image and build a mental model of the surrounding environment. The visual system is associated with the eye and functionally divided into the optical system and the neural system.

In neuroanatomy, the superior colliculus is a structure lying on the roof of the mammalian midbrain. In non-mammalian vertebrates, the homologous structure is known as the optic tectum or optic lobe. The adjective form tectal is commonly used for both structures.

Fish locomotion is the various types of animal locomotion used by fish, principally by swimming. This is achieved in different groups of fish by a variety of mechanisms of propulsion, most often by wave-like lateral flexions of the fish's body and tail in the water, and in various specialised fish by motions of the fins. The major forms of locomotion in fish are:

Motion perception is the process of inferring the speed and direction of elements in a scene based on visual, vestibular and proprioceptive inputs. Although this process appears straightforward to most observers, it has proven to be a difficult problem from a computational perspective, and difficult to explain in terms of neural processing.
Neural adaptation or sensory adaptation is a gradual decrease over time in the responsiveness of the sensory system to a constant stimulus. It is usually experienced as a change in the stimulus. For example, if a hand is rested on a table, the table's surface is immediately felt against the skin. Subsequently, however, the sensation of the table surface against the skin gradually diminishes until it is virtually unnoticeable. The sensory neurons that initially respond are no longer stimulated to respond; this is an example of neural adaptation.

Johnston's organ is a collection of sensory cells found in the pedicel of the antennae in the class Insecta. Johnston's organ detects motion in the flagellum. It consists of scolopidia arrayed in a bowl shape, each of which contains a mechanosensory chordotonal neuron. The number of scolopidia varies between species. In homopterans, the Johnston's organs contain 25–79 scolopidia. The presence of Johnston's organ is a defining characteristic which separates the class Insecta from the other hexapods belonging to the group Entognatha. Johnston's organ was named after the physician Christopher Johnston (1822-1891) father of the physician and Assyriologist Christopher Johnston.

Campaniform sensilla are a class of mechanoreceptors found in insects, which respond to local stress and strain within the animal's cuticle. Campaniform sensilla function as proprioceptors that detect mechanical load as resistance to muscle contraction, similar to mammalian Golgi tendon organs. Sensory feedback from campaniform sensilla is integrated in the control of posture and locomotion.
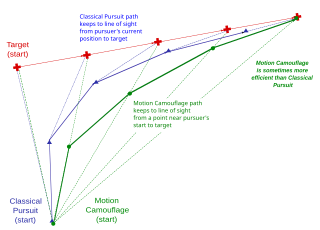
Motion camouflage is camouflage which provides a degree of concealment for a moving object, given that motion makes objects easy to detect however well their coloration matches their background or breaks up their outlines.

The optokinetic reflex (OKR), also referred to as the optokinetic response, or optokinetic nystagmus (OKN), is a compensatory reflex that supports visual image stabilization. The purpose of OKR is to prevent motion blur on the retina that would otherwise occur when an animal moves its head or navigates through its environment. This is achieved by the reflexive movement of the eyes in the same direction as image motion, so as to minimize the relative motion of the visual scene on the eye. OKR is best evoked by slow, rotational motion, and operates in coordination with several complementary reflexes that also support image stabilization, including the vestibulo-ocular reflex (VOR).

Vision is the most important sense for birds, since good eyesight is essential for safe flight. Birds have a number of adaptations which give visual acuity superior to that of other vertebrate groups; a pigeon has been described as "two eyes with wings". Birds are theropods, and the avian eye resembles that of other sauropsids, with ciliary muscles that can change the shape of the lens rapidly and to a greater extent than in the mammals. Birds have the largest eyes relative to their size in the animal kingdom, and movement is consequently limited within the eye's bony socket. In addition to the two eyelids usually found in vertebrates, bird's eyes are protected by a third transparent movable membrane. The eye's internal anatomy is similar to that of other vertebrates, but has a structure, the pecten oculi, unique to birds.
The H1 neuron is located in the visual cortex of true flies of the order Diptera and mediates motor responses to visual stimuli. H1 is sensitive to horizontal motion in the visual field and enables the fly to rapidly and accurately respond to optic flow with motor corrections to stabilize flight. It is particularly responsive to horizontal forward motion associated with movement of the fly's own body during flight. Damage to H1 impairs the fly's ability to counteract disturbances during flight, suggesting that it is a necessary component of the optomotor response. H1 is an ideal system for studying the neural basis of information processing due to its highly selective and predictable responses to stimuli. Since the initial anatomical and physiological characterizations of H1 in 1976, study of the neuron has greatly benefited the understanding of neural coding in a wide range of organisms, especially the relationship between the neural code and behavior.
Ralph Mitchell Siegel, a researcher who studied the neurological underpinnings of vision, was a professor of neuroscience at Rutgers University, Newark, in the Center for Molecular and Behavioral Neuroscience. He died September 2, 2011, at his home following a long illness.
Biological motion perception is the act of perceiving the fluid unique motion of a biological agent. The phenomenon was first documented by Swedish perceptual psychologist, Gunnar Johansson, in 1973. There are many brain areas involved in this process, some similar to those used to perceive faces. While humans complete this process with ease, from a computational neuroscience perspective there is still much to be learned as to how this complex perceptual problem is solved. One tool which many research studies in this area use is a display stimuli called a point light walker. Point light walkers are coordinated moving dots that simulate biological motion in which each dot represents specific joints of a human performing an action.
In visual perception, structure from motion (SFM) refers to how humans recover depth structure from object's motion. The human visual field has an important function: capturing the three-dimensional structures of an object using different kinds of visual cues.
James "Jim" William Truman is an American chronobiologist known for his seminal research on circadian rhythms in silkmoth (Saturniidae) eclosion, particularly the restoration of rhythm and phase following brain transplantation. He is a professor emeritus at the University of Washington and a former senior fellow at Howard Hughes Medical Institution Janelia Research Campus.
Hair plates are a type of proprioceptor found in the folds of insect joints. They consist of a cluster of hairs, in which each hair is innervated by a single mechanosensory neuron. Functionally, hair plates operate as "limit-detectors" by signaling the extremes of joint movement, which then drives reflexive leg movement.
The study of animal locomotion is a branch of biology that investigates and quantifies how animals move.











