Related Research Articles
Collagen is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin.

Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation.
Osteoblasts are cells with a single nucleus that synthesize bone. However, in the process of bone formation, osteoblasts function in groups of connected cells. Individual cells cannot make bone. A group of organized osteoblasts together with the bone made by a unit of cells is usually called the osteon.
A biogenic amine is a biogenic substance with one or more amine groups. They are basic nitrogenous compounds formed mainly by decarboxylation of amino acids or by amination and transamination of aldehydes and ketones. Biogenic amines are organic bases with low molecular weight and are synthesized by microbial, vegetable and animal metabolisms. In food and beverages they are formed by the enzymes of raw material or are generated by microbial decarboxylation of amino acids.
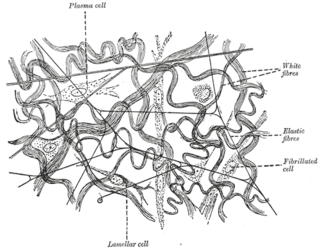
Elastic fibers are an essential component of the extracellular matrix composed of bundles of proteins (elastin) which are produced by a number of different cell types including fibroblasts, endothelial, smooth muscle, and airway epithelial cells. These fibers are able to stretch many times their length, and snap back to their original length when relaxed without loss of energy. Elastic fibers include elastin, elaunin and oxytalan.
Neurolathyrism, is a neurological disease of humans, caused by eating certain legumes of the genus Lathyrus. This disease is mainly associated with the consumption of Lathyrus sativus and to a lesser degree with Lathyrus cicera, Lathyrus ochrus and Lathyrus clymenum containing the toxin ODAP.

The sweet pea, Lathyrus odoratus, is a flowering plant in the genus Lathyrus in the family Fabaceae (legumes), native to Sicily, southern Italy and the Aegean Islands.
Lysyl oxidase (LOX), also known as protein-lysine 6-oxidase, is an enzyme that, in humans, is encoded by the LOX gene. It catalyzes the conversion of lysine molecules into highly reactive aldehydes that form cross-links in extracellular matrix proteins. Its inhibition can cause osteolathyrism, but, at the same time, its upregulation by tumor cells may promote metastasis of the existing tumor, causing it to become malignant and cancerous.
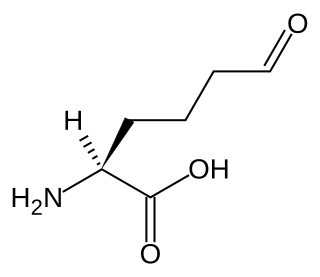
Allysine is a derivative of lysine, used in the production of elastin and collagen. It is produced by the actions of the enzyme lysyl oxidase in the extracellular matrix and is essential in the crosslink formation that stabilizes collagen and elastin.

Aldehyde oxidase (AO) is a metabolizing enzyme, located in the cytosolic compartment of tissues in many organisms. AO catalyzes the oxidation of aldehydes into carboxylic acid, and in addition, catalyzes the hydroxylation of some heterocycles. It can also catalyze the oxidation of both cytochrome P450 (CYP450) and monoamine oxidase (MAO) intermediate products. AO plays an important role in the metabolism of several drugs.

Amine oxidase (copper-containing) (AOC) (EC 1.4.3.21 and EC 1.4.3.22; formerly EC 1.4.3.6) is a family of amine oxidase enzymes which includes both primary-amine oxidase and diamine oxidase; these enzymes catalyze the oxidation of a wide range of biogenic amines including many neurotransmitters, histamine and xenobiotic amines. They act as a disulphide-linked homodimer. They catalyse the oxidation of primary amines to aldehydes, with the subsequent release of ammonia and hydrogen peroxide, which requires one copper ion per subunit and topaquinone as cofactor:

Lysyl oxidase homolog 2 is an enzyme that in humans is encoded by the LOXL2 gene.

Lysyl oxidase homolog 1, also known as LOXL1, is an enzyme which in humans is encoded by the LOXL1 gene.

Lysyl oxidase homolog 4 is an enzyme that in humans is encoded by the LOXL4 gene.

Lysyl oxidase homolog 3 is an enzyme that in humans is encoded by the LOXL3 gene.
Aminopropionitrile, also known as β-aminopropionitrile (BAPN), is an organic compound with both amine and nitrile functional groups. It is a colourless liquid. The compound occurs naturally and is of interest in the biomedical community.

Pyridinoline, also known as Hydroxylysylpyridinoline, is a fluorescent cross-linking compound of collagen fibers. Crosslinks in collagen and elastin are derived from lysyl and hydroxylysyl residues, a process catalyzed by lysyl oxidase. Fujimoto and colleagues first described the isolation and characterization of a fluorescent material in bovine Achilles tendon collagen and termed it pyridinoline. It is reported to be present in collagen of bone and cartilage, but is absent in collagen of skin. It is not present in newly synthesized collagen and is formed from aldimine cross-links during maturation of collagen fibers.
Angiolathyrism is a form of Lathyrism disease. It is mainly caused the consumption of Lathyrus sativus and to a lesser degree by Lathyrus cicera, Lathyrus ochrus and Lathyrus clymenum containing the toxin ODAP. The main chemical responsible is β-Aminopropionitrile, which prevents collagen cross-linking, thus making the blood vessel, especially the tunica media, weak. This can result in Cystic medial necrosis or a picture similar to Marfan syndrome. The damaged vessels are at an increased risk of dissection.

Primary-amine oxidase, also known as semicarbazide-sensitive amine oxidase (SSAO), is an enzyme (EC 1.4.3.21) with the systematic name primary-amine:oxygen oxidoreductase (deaminating). This enzyme catalyses the following chemical reaction
Lathyrism is a condition caused by eating certain legumes of the genus Lathyrus. There are three types of lathyrism: neurolathyrism, osteolathyrism, and angiolathyrism, all of which are incurable, differing in their symptoms and in the body tissues affected.
References
- 1 2 Dasler, Waldemar; Mosby, Mildred (November 1954). "Incisor Ash Versus Femur Ash in Sweet Pea Lathyrism (Odoratism)". The Journal of Nutrition. 54 (3): 397–402. doi:10.1093/jn/54.3.397. PMID 13212476.
- 1 2 3 4 Haque, Abdul; Hossain, Muffazal; Lambien, Fernand; Bell, E. Arthur (May 2006). "Evidence of Osteolathyrism among patients suffering from Neurolathyrism in Bangladesh". Natural Toxins. 5 (1): 43–6. doi:10.1002/(SICI)(1997)5:1<43::AID-NT7>3.0.CO;2-M. PMID 9086459.
- 1 2 3 4 Rosenthal, Gerald (2003). "Toxic Constituents and their Related Metabolites". Plant Nonprotein Amino and Imino Acids: Biological, Biochemical, and Toxicological Properties. Elsevier. ISBN 9780323157742.
- ↑ Wilmarth, K. R.; Froines, J. R. (1992). "In vitro and in vivo inhibition of lysyl oxidase by aminopropionitriles". Journal of Toxicology and Environmental Health. 37 (3): 411–23. doi:10.1080/15287399209531680. PMID 1359158.
- ↑ Dawson, D. A.; Rinaldi, A. C.; Pöch, G. (2002). "Biochemical and toxicological evaluation of agent-cofactor reactivity as a mechanism of action for osteolathyrism". Toxicology. 177 (2–3): 267–84. doi:10.1016/s0300-483x(02)00233-0. PMID 12135629.
- ↑ Bailey, A. J.; Peach, C. M. (1971). "The chemistry of the collagen cross-links. The absence of reduction of dehydrolysinonorleucine and dehydrohydroxylysinonorleucine in vivo". The Biochemical Journal. 121 (2): 257–9. doi:10.1042/bj1210257. PMC 1176564 . PMID 5117030.