In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

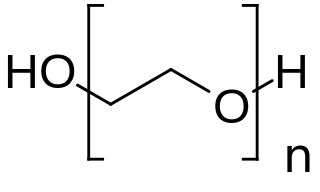
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular weight. The structure of PEG is commonly expressed as H−(O−CH2−CH2)n−OH.

Laxatives, purgatives, or aperients are substances that loosen stools and increase bowel movements. They are used to treat and prevent constipation.

Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, and thermoforming for manufacturing, and in combination with glass fibre for engineering resins.
Excipient is a substance formulated alongside the active ingredient of a medication. Excipients serve various purposes, including long-term stabilization, bulking up solid formulations containing potent active ingredients in small amounts, or enhancing the therapeutic properties of the active ingredient in the final dosage form. They can facilitate drug absorption, reduce viscosity, or enhance solubility. Excipients can also aid in the manufacturing process by improving the handling of active substances, facilitating powder flowability, or preventing denaturation and aggregation during the expected shelf life. The selection of excipients depends on factors such as the route of administration, dosage form, and active ingredient.

Low-density polyethylene (LDPE) is a thermoplastic made from the monomer ethylene. It was the first grade of polyethylene, produced in 1933 by Dr John C. Swallow and M.W Perrin who were working for Imperial Chemical Industries (ICI) using a high pressure process via free radical polymerization. Its manufacture employs the same method today. The EPA estimates 5.7% of LDPE is recycled in the United States. Despite competition from more modern polymers, LDPE continues to be an important plastic grade. In 2013 the worldwide LDPE market reached a volume of about US$33 billion.
In biotechnology, polymersomes are a class of artificial vesicles, tiny hollow spheres that enclose a solution. Polymersomes are made using amphiphilic synthetic block copolymers to form the vesicle membrane, and have radii ranging from 50 nm to 5 μm or more. Most reported polymersomes contain an aqueous solution in their core and are useful for encapsulating and protecting sensitive molecules, such as drugs, enzymes, other proteins and peptides, and DNA and RNA fragments. The polymersome membrane provides a physical barrier that isolates the encapsulated material from external materials, such as those found in biological systems.
Aqueous biphasic systems (ABS) or aqueous two-phase systems (ATPS) are clean alternatives for traditional organic-water solvent extraction systems.

An amphiphile, or amphipath, is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Amphiphilic compounds include surfactants and detergents. The phospholipid amphiphiles are the major structural component of cell membranes.

Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
A multiphasic liquid is a mixture consisting of more than two immiscible liquid phases. Biphasic mixtures consisting of two immiscible phases are very common and usually consist of an organic solvent and an aqueous phase.
Poloxamer 407 is a hydrophilic non-ionic surfactant of the more general class of copolymers known as poloxamers. Poloxamer 407 is a triblock copolymer consisting of a central hydrophobic block of polypropylene glycol flanked by two hydrophilic blocks of polyethylene glycol (PEG). The approximate lengths of the two PEG blocks is 101 repeat units, while the approximate length of the propylene glycol block is 56 repeat units. This particular compound is also known by the BASF trade name Pluronic F-127 or by the Croda trade name Synperonic PE/F 127. BASF also offers a pharmaceutical grade, under trade name Kolliphor P 407.
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.

Macrogol, also known as polyethylene glycol (PEG), is used as a medication to treat constipation in children and adults. It is taken by mouth. Benefits usually occur within three days. Generally it is only recommended for up to two weeks. It is also used as an excipient. It is also used to clear the bowels before a colonoscopy, when the onset of the laxative effect is more rapid, typically within an hour.
Polypure is a Norwegian company that manufactures and markets monodisperse PEG derivatives for applications in nanotechnology, biotechnology and in pharmaceutical sciences.
Ultra-low fouling is a rating of a surface's ability to shed potential contamination. Surfaces are prone to contamination, which is a phenomenon known as fouling. Unwanted adsorbates caused by fouling change the properties of a surface, which is often counter-productive to the function of that surface. Consequently, a necessity for anti-fouling surfaces has arisen in many fields: blocked pipes inhibit factory productivity, biofouling increases fuel consumption on ships, medical devices must be kept sanitary, etc. Although chemical fouling inhibitors, metallic coatings, and cleaning processes can be used to reduce fouling, non-toxic surfaces with anti-fouling properties are ideal for fouling prevention. To be considered effective, an ultra-low fouling surface must be able to repel and withstand the accumulation of detrimental aggregates down to less than 5 ng/cm2. A recent surge of research has been conducted to create these surfaces in order to benefit the biological, nautical, mechanical, and medical fields.

Self-healing hydrogels are a specialized type of polymer hydrogel. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are synthesized from hydrophilic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhance the hydrogel's ability to absorb large amounts of water via hydrogen bonding. As a result, hydrogels, self-healing alike, develop characteristic firm yet elastic mechanical properties. Self-healing refers to the spontaneous formation of new bonds when old bonds are broken within a material. The structure of the hydrogel along with electrostatic attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties have motivated the research and development of self-healing hydrogels in fields such as reconstructive tissue engineering as scaffolding, as well as use in passive and preventive applications.
Lysozyme PEGylation is the covalent attachment of Polyethylene glycol (PEG) to Lysozyme, which is one of the most widely investigated PEGylated proteins.












