Histone methyltransferases (HMT) are histone-modifying enzymes, that catalyze the transfer of one, two, or three methyl groups to lysine and arginine residues of histone proteins. The attachment of methyl groups occurs predominantly at specific lysine or arginine residues on histones H3 and H4. Two major types of histone methyltranferases exist, lysine-specific and arginine-specific. In both types of histone methyltransferases, S-Adenosyl methionine (SAM) serves as a cofactor and methyl donor group.
The genomic DNA of eukaryotes associates with histones to form chromatin. The level of chromatin compaction depends heavily on histone methylation and other post-translational modifications of histones. Histone methylation is a principal epigenetic modification of chromatin that determines gene expression, genomic stability, stem cell maturation, cell lineage development, genetic imprinting, DNA methylation, and cell mitosis.

Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-N-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression.
Histone H4 is one of the five main histone proteins involved in the structure of chromatin in eukaryotic cells. Featuring a main globular domain and a long N-terminal tail, H4 is involved with the structure of the nucleosome of the 'beads on a string' organization. Histone proteins are highly post-translationally modified. Covalently bonded modifications include acetylation and methylation of the N-terminal tails. These modifications may alter expression of genes located on DNA associated with its parent histone octamer. Histone H4 is an important protein in the structure and function of chromatin, where its sequence variants and variable modification states are thought to play a role in the dynamic and long term regulation of genes.

Histone acetyltransferase p300 also known as p300 HAT or E1A-associated protein p300 also known as EP300 or p300 is an enzyme that, in humans, is encoded by the EP300 gene. It functions as histone acetyltransferase that regulates transcription of genes via chromatin remodeling. This enzyme plays an essential role in regulating cell growth and division, prompting cells to mature and assume specialized functions (differentiate), and preventing the growth of cancerous tumors. The p300 protein appears to be critical for normal development before and after birth.
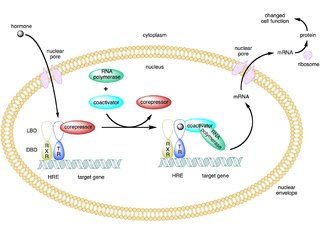
A coactivator is a type of transcriptional coregulator that binds to an activator to increase the rate of transcription of a gene or set of genes. The activator contains a DNA binding domain that binds either to a DNA promoter site or a specific DNA regulatory sequence called an enhancer. Binding of the activator-coactivator complex increases the speed of transcription by recruiting general transcription machinery to the promoter, therefore increasing gene expression. The use of activators and coactivators allows for highly specific expression of certain genes depending on cell type and developmental stage.
Histone methylation is a process by which methyl groups are transferred to amino acids of histone proteins that make up nucleosomes, which the DNA double helix wraps around to form chromosomes. Methylation of histones can either increase or decrease transcription of genes, depending on which amino acids in the histones are methylated, and how many methyl groups are attached. Methylation events that weaken chemical attractions between histone tails and DNA increase transcription, because they enable the DNA to uncoil from nucleosomes so that transcription factor proteins and RNA polymerase can access the DNA. This process is critical for the regulation of gene expression that allows different cells to express different genes.
In molecular biology and genetics, transcription coregulators are proteins that interact with transcription factors to either activate or repress the transcription of specific genes. Transcription coregulators that activate gene transcription are referred to as coactivators while those that repress are known as corepressors. The mechanism of action of transcription coregulators is to modify chromatin structure and thereby make the associated DNA more or less accessible to transcription. In humans several dozen to several hundred coregulators are known, depending on the level of confidence with which the characterisation of a protein as a coregulator can be made. One class of transcription coregulators modifies chromatin structure through covalent modification of histones. A second ATP dependent class modifies the conformation of chromatin.

P300/CBP-associated factor (PCAF), also known as K(lysine) acetyltransferase 2B (KAT2B), is a human gene and transcriptional coactivator associated with p53.
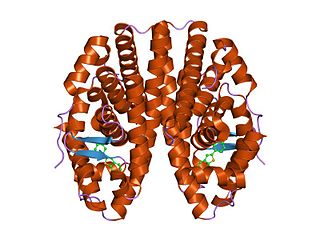
The nuclear receptor coactivator 2 also known as NCoA-2 is a protein that in humans is encoded by the NCOA2 gene. NCoA-2 is also frequently called glucocorticoid receptor-interacting protein 1 (GRIP1), steroid receptor coactivator-2 (SRC-2), or transcriptional mediators/intermediary factor 2 (TIF2).

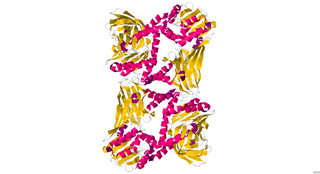
The nuclear receptor coactivator 3 also known as NCOA3 is a protein that, in humans, is encoded by the NCOA3 gene. NCOA3 is also frequently called 'amplified in breast 1' (AIB1), steroid receptor coactivator-3 (SRC-3), or thyroid hormone receptor activator molecule 1 (TRAM-1).

The nuclear receptor co-repressor 1 also known as thyroid-hormone- and retinoic-acid-receptor-associated co-repressor 1 (TRAC-1) is a protein that in humans is encoded by the NCOR1 gene.
Chromatin remodeling is the dynamic modification of chromatin architecture to allow access of condensed genomic DNA to the regulatory transcription machinery proteins, and thereby control gene expression. Such remodeling is principally carried out by 1) covalent histone modifications by specific enzymes, e.g., histone acetyltransferases (HATs), deacetylases, methyltransferases, and kinases, and 2) ATP-dependent chromatin remodeling complexes which either move, eject or restructure nucleosomes. Besides actively regulating gene expression, dynamic remodeling of chromatin imparts an epigenetic regulatory role in several key biological processes, egg cells DNA replication and repair; apoptosis; chromosome segregation as well as development and pluripotency. Aberrations in chromatin remodeling proteins are found to be associated with human diseases, including cancer. Targeting chromatin remodeling pathways is currently evolving as a major therapeutic strategy in the treatment of several cancers.
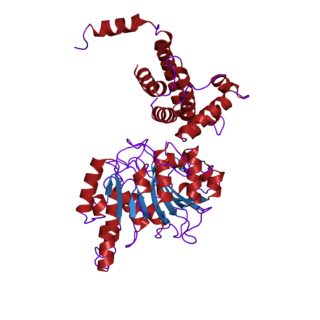
CARM1, also known as PRMT4, is an enzyme encoded by the CARM1 gene found in human beings, as well as many other mammals. It has a polypeptide (L) chain type that is 348 residues long, and is made up of alpha helices and beta sheets. Its main function includes catalyzing the transfer of a methyl group from S-adenosyl-L-methionine to the side chain nitrogens of arginine residues within proteins to form methylated arginine derivatives and S-adenosyl-L-homocysteine. CARM1 is a secondary coactivator through its association with p160 family of coactivators. It is responsible for moving cells toward the inner cell mass in developing blastocysts.
Protein arginine N-methyltransferase 1 is an enzyme that in humans is encoded by the PRMT1 gene.

Protein arginine N-methyltransferase 5 is an enzyme that in humans is encoded by the PRMT5 gene. PRMT5 symmetrically dimethylates H2AR3, H4R3, H3R2, and H3R8 in vivo, all of which are linked to a range of transcriptional regulatory events

Nuclear receptor coactivator 6 is a protein that in humans is encoded by the NCOA6 gene.

Protein arginine N-methyltransferase 6 is an enzyme that in humans is encoded by the PRMT6 gene.
Nuclear receptor coregulators are a class of transcription coregulators that have been shown to be involved in any aspect of signaling by any member of the nuclear receptor superfamily. A comprehensive database of nuclear receptor coregulators can be found at the Nuclear Receptor Signaling Atlas website.
Protein methylation is a type of post-translational modification featuring the addition of methyl groups to proteins. It can occur on the nitrogen-containing side-chains of arginine and lysine, but also at the amino- and carboxy-termini of a number of different proteins. In biology, methyltransferases catalyze the methylation process, activated primarily by S-adenosylmethionine. Protein methylation has been most studied in histones, where the transfer of methyl groups from S-adenosyl methionine is catalyzed by histone methyltransferases. Histones that are methylated on certain residues can act epigenetically to repress or activate gene expression.