
Arabidopsis thaliana, the thale cress, mouse-ear cress or arabidopsis, is a small plant from the mustard family (Brassicaceae), native to Eurasia and Africa. Commonly found along the shoulders of roads and in disturbed land, it is generally considered a weed.
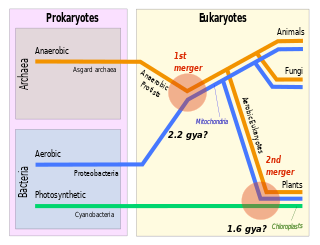
Symbiogenesis is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms. The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells are descended from formerly free-living prokaryotes taken one inside the other in endosymbiosis. Mitochondria appear to be phylogenetically related to Rickettsiales bacteria, while chloroplasts are thought to be related to cyanobacteria.
A plastid is a membrane-bound organelle found in the cells of plants, algae, and some other eukaryotic organisms. They are considered to be intracellular endosymbiotic cyanobacteria.

Horizontal gene transfer (HGT) or lateral gene transfer (LGT) is the movement of genetic material between organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction). HGT is an important factor in the evolution of many organisms. HGT is influencing scientific understanding of higher-order evolution while more significantly shifting perspectives on bacterial evolution.

In molecular biology and genetics, transformation is the genetic alteration of a cell resulting from the direct uptake and incorporation of exogenous genetic material from its surroundings through the cell membrane(s). For transformation to take place, the recipient bacterium must be in a state of competence, which might occur in nature as a time-limited response to environmental conditions such as starvation and cell density, and may also be induced in a laboratory.

Agrobacterium is a genus of Gram-negative bacteria established by H. J. Conn that uses horizontal gene transfer to cause tumors in plants. Agrobacterium tumefaciens is the most commonly studied species in this genus. Agrobacterium is well known for its ability to transfer DNA between itself and plants, and for this reason it has become an important tool for genetic engineering.

In genetic engineering, a gene gun or biolistic particle delivery system is a device used to deliver exogenous DNA (transgenes), RNA, or protein to cells. By coating particles of a heavy metal with a gene of interest and firing these micro-projectiles into cells using mechanical force, an integration of desired genetic information can be introduced into desired cells. The technique involved with such micro-projectile delivery of DNA is often referred to as biolistics, short for "biological ballistics".

A nuclear gene is a gene that has its DNA nucleotide sequence physically situated within the cell nucleus of a eukaryotic organism. This term is employed to differentiate nuclear genes, which are located in the cell nucleus, from genes that are found in mitochondria or chloroplasts. The vast majority of genes in eukaryotes are nuclear.
Extranuclear inheritance or cytoplasmic inheritance is the transmission of genes that occur outside the nucleus. It is found in most eukaryotes and is commonly known to occur in cytoplasmic organelles such as mitochondria and chloroplasts or from cellular parasites like viruses or bacteria.

Gene delivery is the process of introducing foreign genetic material, such as DNA or RNA, into host cells. Gene delivery must reach the genome of the host cell to induce gene expression. Successful gene delivery requires the foreign gene delivery to remain stable within the host cell and can either integrate into the genome or replicate independently of it. This requires foreign DNA to be synthesized as part of a vector, which is designed to enter the desired host cell and deliver the transgene to that cell's genome. Vectors utilized as the method for gene delivery can be divided into two categories, recombinant viruses and synthetic vectors.

Agroinfiltration is a method used in plant biology and especially lately in plant biotechnology to induce transient expression of genes in a plant, or isolated leaves from a plant, or even in cultures of plant cells, in order to produce a desired protein. In the method, a suspension of Agrobacterium tumefaciens is introduced into a plant leaf by direct injection or by vacuum infiltration, or brought into association with plant cells immobilised on a porous support, whereafter the bacteria transfer the desired gene into the plant cells via transfer of T-DNA. The main benefit of agroinfiltration when compared to the more traditional plant transformation is speed and convenience, although yields of the recombinant protein are generally also higher and more consistent.

A transplastomic plant is a genetically modified plant in which genes are inactivated, modified or new foreign genes are inserted into the DNA of plastids like the chloroplast instead of nuclear DNA.
A transfer DNA (T-DNA) binary system is a pair of plasmids consisting of a T-DNA binary vector and a virhelper plasmid. The two plasmids are used together to produce genetically modified plants. They are artificial vectors that have been derived from the naturally occurring Ti plasmid found in bacterial species of the genus Agrobacterium, such as A. tumefaciens. The binary vector is a shuttle vector, so-called because it is able to replicate in multiple hosts.

Plant genetics is the study of genes, genetic variation, and heredity specifically in plants. It is generally considered a field of biology and botany, but intersects frequently with many other life sciences and is strongly linked with the study of information systems. Plant genetics is similar in many ways to animal genetics but differs in a few key areas.
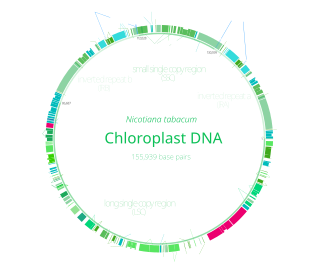
Chloroplast DNA (cpDNA) is the DNA located in chloroplasts, which are photosynthetic organelles located within the cells of some eukaryotic organisms. Chloroplasts, like other types of plastid, contain a genome separate from that in the cell nucleus. The existence of chloroplast DNA was identified biochemically in 1959, and confirmed by electron microscopy in 1962. The discoveries that the chloroplast contains ribosomes and performs protein synthesis revealed that the chloroplast is genetically semi-autonomous. The first complete chloroplast genome sequences were published in 1986, Nicotiana tabacum (tobacco) by Sugiura and colleagues and Marchantia polymorpha (liverwort) by Ozeki et al. Since then, a great number of chloroplast DNAs from various species have been sequenced.

Genetic engineering is the science of manipulating genetic material of an organism. The first artificial genetic modification accomplished using biotechnology was transgenesis, the process of transferring genes from one organism to another, first accomplished by Herbert Boyer and Stanley Cohen in 1973. It was the result of a series of advancements in techniques that allowed the direct modification of the genome. Important advances included the discovery of restriction enzymes and DNA ligases, the ability to design plasmids and technologies like polymerase chain reaction and sequencing. Transformation of the DNA into a host organism was accomplished with the invention of biolistics, Agrobacterium-mediated recombination and microinjection. The first genetically modified animal was a mouse created in 1974 by Rudolf Jaenisch. In 1976 the technology was commercialised, with the advent of genetically modified bacteria that produced somatostatin, followed by insulin in 1978. In 1983 an antibiotic resistant gene was inserted into tobacco, leading to the first genetically engineered plant. Advances followed that allowed scientists to manipulate and add genes to a variety of different organisms and induce a range of different effects. Plants were first commercialized with virus resistant tobacco released in China in 1992. The first genetically modified food was the Flavr Savr tomato marketed in 1994. By 2010, 29 countries had planted commercialized biotech crops. In 2000 a paper published in Science introduced golden rice, the first food developed with increased nutrient value.

Genetic engineering techniques allow the modification of animal and plant genomes. Techniques have been devised to insert, delete, and modify DNA at multiple levels, ranging from a specific base pair in a specific gene to entire genes. There are a number of steps that are followed before a genetically modified organism (GMO) is created. Genetic engineers must first choose what gene they wish to insert, modify, or delete. The gene must then be isolated and incorporated, along with other genetic elements, into a suitable vector. This vector is then used to insert the gene into the host genome, creating a transgenic or edited organism.
A plastid is a membrane-bound organelle found in plants, algae and other eukaryotic organisms that contribute to the production of pigment molecules. Most plastids are photosynthetic, thus leading to color production and energy storage or production. There are many types of plastids in plants alone, but all plastids can be separated based on the number of times they have undergone endosymbiotic events. Currently there are three types of plastids; primary, secondary and tertiary. Endosymbiosis is reputed to have led to the evolution of eukaryotic organisms today, although the timeline is highly debated.

Maureen Hanson is an American molecular biologist and Liberty Hyde Bailey Professor in the Department of Molecular Biology and Genetics at Cornell University in Ithaca, New York. She is a joint member of the Section of Plant Biology and Director of the Center for Enervating Neuroimmune Disease. Her research concerns gene expression in chloroplasts and mitochondria, photosynthesis, and the molecular basis of the disease Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).














