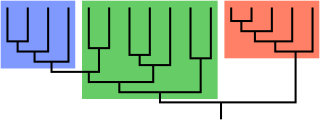
In biological phylogenetics, a clade, also known as a monophyletic group or natural group, is a grouping of organisms that are monophyletic – that is, composed of a common ancestor and all its lineal descendants – on a phylogenetic tree. In the taxonomical literature, sometimes the Latin form cladus is used rather than the English form. Clades are the fundamental unit of cladistics, a modern approach to taxonomy adopted by most biological fields.
Macroevolution usually means the evolution of large-scale structures and traits that go significantly beyond the intraspecific variation found in microevolution. In other words, macroevolution is the evolution of taxa above the species level.

Lobopodians are members of the informal group Lobopodia, or the formally erected phylum Lobopoda Cavalier-Smith (1998). They are panarthropods with stubby legs called lobopods, a term which may also be used as a common name of this group as well. While the definition of lobopodians may differ between literatures, it usually refers to a group of soft-bodied, marine worm-like fossil panarthropods such as Aysheaia and Hallucigenia.

Evolutionary developmental biology is a field of biological research that compares the developmental processes of different organisms to infer how developmental processes evolved.

Panarthropoda is a proposed animal clade containing the extant phyla Arthropoda, Tardigrada and Onychophora. Panarthropods also include extinct marine legged worms known as lobopodians ("Lobopodia"), a paraphyletic group where the last common ancestor and basal members (stem-group) of each extant panarthropod phylum are thought to have risen. However the term "Lobopodia" is sometimes expanded to include tardigrades and onychophorans as well.

Morphometrics or morphometry refers to the quantitative analysis of form, a concept that encompasses size and shape. Morphometric analyses are commonly performed on organisms, and are useful in analyzing their fossil record, the impact of mutations on shape, developmental changes in form, covariances between ecological factors and shape, as well for estimating quantitative-genetic parameters of shape. Morphometrics can be used to quantify a trait of evolutionary significance, and by detecting changes in the shape, deduce something of their ontogeny, function or evolutionary relationships. A major objective of morphometrics is to statistically test hypotheses about the factors that affect shape.

Carcinisation is a form of convergent evolution in which non-crab crustaceans evolve a crab-like body plan. The term was introduced into evolutionary biology by L. A. Borradaile, who described it as "the many attempts of Nature to evolve a crab".
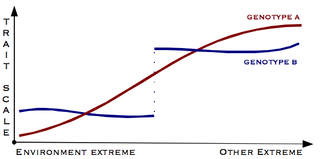
Canalisation is a measure of the ability of a population to produce the same phenotype regardless of variability of its environment or genotype. It is a form of evolutionary robustness. The term was coined in 1942 by C. H. Waddington to capture the fact that "developmental reactions, as they occur in organisms submitted to natural selection...are adjusted so as to bring about one definite end-result regardless of minor variations in conditions during the course of the reaction". He used this word rather than robustness to consider that biological systems are not robust in quite the same way as, for example, engineered systems.
Plicatoperipatus is a monospecific genus of velvet worm containing the single species Plicatoperipatus jamaicensis. It is endemic to Jamaica. Females of this species can have as many as 43 pairs of legs, the maximum number found in the phylum Onychophora. In a large sample collected in 1988, however, females ranged from 35 to 39 leg pairs, with 37 as the mean and the most common number, and males ranged from 31 to 37 leg pairs, with 35 as the mean and the most common number. This species ranges from 25 mm to 65 mm in length. In the 1988 sample, the mean length for males was 33 mm, and the mean length for mature females was 51 mm. This species is viviparous, with mothers supplying nourishment to their embryos through a placenta.

Marine invertebrates are the invertebrates that live in marine habitats. Invertebrate is a blanket term that includes all animals apart from the vertebrate members of the chordate phylum. Invertebrates lack a vertebral column, and some have evolved a shell or a hard exoskeleton. As on land and in the air, marine invertebrates have a large variety of body plans, and have been categorised into over 30 phyla. They make up most of the macroscopic life in the oceans.

Global biodiversity is the measure of biodiversity on planet Earth and is defined as the total variability of life forms. More than 99 percent of all species that ever lived on Earth are estimated to be extinct. Estimates on the number of Earth's current species range from 2 million to 1 trillion, but most estimates are around 11 million species or fewer. About 1.74 million species were databased as of 2018, and over 80 percent have not yet been described. The total amount of DNA base pairs on Earth, as a possible approximation of global biodiversity, is estimated at 5.0 x 1037, and weighs 50 billion tonnes. In comparison, the total mass of the biosphere has been estimated to be as much as 4 TtC (trillion tons of carbon).

In biology, a phylum is a level of classification or taxonomic rank below kingdom and above class. Traditionally, in botany the term division has been used instead of phylum, although the International Code of Nomenclature for algae, fungi, and plants accepts the terms as equivalent. Depending on definitions, the animal kingdom Animalia contains about 31 phyla, the plant kingdom Plantae contains about 14 phyla, and the fungus kingdom Fungi contains about 8 phyla. Current research in phylogenetics is uncovering the relationships among phyla within larger clades like Ecdysozoa and Embryophyta.

A species is often defined as the largest group of organisms in which any two individuals of the appropriate sexes or mating types can produce fertile offspring, typically by sexual reproduction. It is the basic unit of classification and a taxonomic rank of an organism, as well as a unit of biodiversity. Other ways of defining species include their karyotype, DNA sequence, morphology, behaviour, or ecological niche. In addition, paleontologists use the concept of the chronospecies since fossil reproduction cannot be examined. The most recent rigorous estimate for the total number of species of eukaryotes is between 8 and 8.7 million. About 14% of these had been described by 2011. All species are given a two-part name, a "binomial". The first part of a binomial is the genus to which the species belongs. The second part is called the specific name or the specific epithet. For example, Boa constrictor is one of the species of the genus Boa, with constrictor being the species' epithet.
A variety of objective means exist to empirically measure biodiversity. Each measure relates to a particular use of the data, and is likely to be associated with the variety of genes. Biodiversity is commonly measured in terms of taxonomic richness of a geographic area over a time interval. In order to calculate biodiversity, species evenness, species richness, and species diversity are to be obtained first. Species evenness is the relative number of individuals of each species in a given area. Species richness is the number of species present in a given area. Species diversity is the relationship between species evenness and species richness. There are many ways to measure biodiversity within a given ecosystem. However, the two most popular are Shannon-Weaver diversity index, commonly referred to as Shannon diversity index, and the other is Simpsons diversity index. Although many scientists prefer to use Shannon's diversity index simply because it takes into account species richness.

The Avalon explosion, named from the Precambrian faunal trace fossils discovered on the Avalon Peninsula in Newfoundland, eastern Canada, is a proposed evolutionary radiation of prehistoric animals about 575 million years ago in the Ediacaran period, with the Avalon explosion being one of three eras grouped in this time period. This evolutionary event is believed to have occurred some 33 million years earlier than the Cambrian explosion, which had been long thought to be when complex life started on Earth.

The following outline is provided as an overview of and topical guide to evolution:
In evolutionary biology, developmental bias refers to the production against or towards certain ontogenetic trajectories which ultimately influence the direction and outcome of evolutionary change by affecting the rates, magnitudes, directions and limits of trait evolution. Historically, the term was synonymous with developmental constraint, however, the latter has been more recently interpreted as referring solely to the negative role of development in evolution.

Collinsovermis is a genus of extinct panarthropod belonging to the group Lobopodia and known from the middle Cambrian Burgess Shale in British Columbia, Canada. It is monotypic having only one species, Collinsovermis monstruosus. After its initial discovery in 1983, Desmond H. Collins popularised it as a unique animal and was subsequently dubbed "Collins' monster" for its unusual super armoured body. The formal scientific description and name were given in 2020.

Acinocricus is a genus of extinct panarthropod belonging to the group Lobopodia and known from the middle Cambrian Spence Shale of Utah, United States. As a monotypic genus, it has one species Acinocricus stichus. The only lobopodian discovered from the Spence Shale, it was described by Simon Conway Morris and Richard A. Robison in 1988. Owing to the original fragmentary fossils discovered since 1982, it was initially classified as an alga, but later realised to be an animal belonging to Cambrian fauna.
Luolishania is an extinct genus of lobopodian panarthropod and known from the Lower Cambrian Chiungchussu Formation of the Chengjiang County, Yunnan Province, China. A monotypic genus, it contains one species Luolishania longicruris. It was discovered and described by Hou Xian-Guang and Chen Jun-Yuan in 1989. It is one of the superarmoured Cambrian lobopodians suspected to be either an intermediate form in the origin of velvet worms (Onychophora) or basal to at least Tardigrada and Arthropoda. It is the basis of the family name Luolishaniidae, which also include other related lobopods such as Acinocricus, Collinsium, Facivermis, and Ovatiovermis. Along with Microdictyon, it is the first lobopodian fossil discovered from China.














