
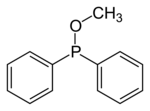
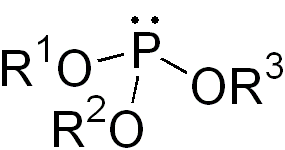
In organic chemistry, phosphinites are organophosphorus compounds with the formula P(OR)R2. They are used as ligands in homogeneous catalysis and coordination chemistry. [1]


In organic chemistry, phosphinites are organophosphorus compounds with the formula P(OR)R2. They are used as ligands in homogeneous catalysis and coordination chemistry. [1]
Phosphinites are prepared by alcoholysis of organophosphinous chlorides. For example, treatment of chlorodiphenylphosphine with methanol and base gives methyl diphenylphosphinite:
Although they are esters of phosphinous acids (R2POH), phosphinites are not made via such intermediates.
Oxidation of phosphinites gives phosphinates:
Phosphinites are ligands, giving derivatives similar to metal phosphine complexes. They are stronger pi-acceptors than typical phosphine ligands. [2]
A phosphite anion or phosphite in inorganic chemistry usually refers to [HPO3]2− but includes [H2PO3]− ([HPO2(OH)]−). These anions are the conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character.
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: Production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and drugs. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.
In chemistry, a transition metal pincer complex is a type of coordination complex with a pincer ligand. Pincer ligands are chelating agents that binds tightly to three adjacent coplanar sites in a meridional configuration. The inflexibility of the pincer-metal interaction confers high thermal stability to the resulting complexes. This stability is in part ascribed to the constrained geometry of the pincer, which inhibits cyclometallation of the organic substituents on the donor sites at each end. In the absence of this effect, cyclometallation is often a significant deactivation process for complexes, in particular limiting their ability to effect C-H bond activation. The organic substituents also define a hydrophobic pocket around the reactive coordination site. Stoichiometric and catalytic applications of pincer complexes have been studied at an accelerating pace since the mid-1970s. Most pincer ligands contain phosphines. Reactions of metal-pincer complexes are localized at three sites perpendicular to the plane of the pincer ligand, although in some cases one arm is hemi-labile and an additional coordination site is generated transiently. Early examples of pincer ligands were anionic with a carbanion as the central donor site and flanking phosphine donors; these compounds are referred to as PCP pincers.
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

The Michaelis–Arbuzov reaction is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. The picture below shows the most common types of substrates undergoing the Arbuzov reaction; phosphite esters (1) react to form phosphonates (2), phosphonites (3) react to form phosphinates (4) and phosphinites (5) react to form phosphine oxides (6).
In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids.
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents.

1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.
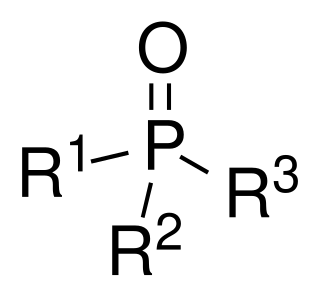
Phosphine oxides are phosphorus compounds with the formula OPX3. When X = alkyl or aryl, these are organophosphine oxides. Triphenylphosphine oxide is an example. An inorganic phosphine oxide is phosphoryl chloride (POCl3).

In organic chemistry, phosphonites are organophosphorus compounds with the formula P(OR)2R. They are found in some pesticides and are used as ligands.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).

Triethyl phosphite is an organophosphorus compound with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis

Triisopropylphosphine is the tertiary phosphine with the formula P(CH(CH3)2)3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to Pi-Pr3 or PiPr3. This ligand is one of the most basic alkyl phosphines with a large ligand cone angle of 160.

Dimethylphenylphosphine is an organophosphorus compound with a formula P(C6H5)(CH3)2. The phosphorus is connected to a phenyl group and two methyl groups, making it the simplest aromatic alkylphosphine. It is colorless air sensitive liquid. It is a member of series (CH3)3-n(C6H5)2P that also includes n = 0, n = 2, and n = 3 that are often employed as ligands in metal phosphine complexes.

Diphenyl-2-pyridylphosphine is an organophosphorus compound with the formula P(C6H5)2(2-C5H4N). It is the most widely used mono-pyridylphosphine ligand. Other mono-pyridylphosphines ligands (3-, 4-) are not common in chemical literature; however, tris-pyridylphosphines have been thoroughly investigated as ligands in transition metal complexes used for catalysis. Pyridylphosphines, including diphenyl-2-pyridylphosphine, may bind transition metals as monodentate or bidentate ligands4. Diphenyl-2-pyridylphosphine behaves as a P-bound monodentate ligand, or a P,N-bound bidentate ligand. Diphenyl-2-pyridylphosphine is a sought after ligand for its ability to relay protons to transition metals such as palladium(II) in homogeneous catalysis.

A metal-phosphine complex is a In coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Vinyldiphenylphosphine is the organophosphorus compound with the formula (C6H5)2PCH=CH2. This colorless, air-sensitive solid is used as a precursor to ligands used in coordination chemistry and homogeneous catalysis. It is prepared by treating chlorodiphenylphosphine with vinyl Grignard reagents.

Diethylphosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethylphosphite is a colorless liquid. The molecule is tetrahedral.
In organophosphorus chemistry, an aminophosphine is a compound with the formula R3−nP(NR2)n where R = H or an organic substituent, and n = 0, 1, 2. At one extreme, the parent H2PNH2 is lightly studied and fragile, but at the other extreme tris(dimethylamino)phosphine (P(NMe2)3) is commonly available. Intermediate members are known, such as Ph2PN(H)Ph. These compounds are typically colorless and reactive toward oxygen. They have pyramidal geometry at phosphorus.

Tris(dimethylamino)phosphine is an organophosphorus compound with the formula P(NMe2)3 (Me = methyl). It is a colorless oil at room temperature, and is one of the most common aminophosphines. Its structure has been determined by X-ray crystallography.